| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 3, June 2025, pages 267-277
Epidemiology and Short-Term Outcomes of Heart Failure With Preserved and Mildly Reduced Ejection Fraction in Colombia: Insights of the Colombian Heart Failure Registry (RECOLFACA)
Lisbeth N. Morales-Rodrigueza, Alex Rivera-Toquicab, c, d, Clara Saldarriagae, Rolando Palaciof, Luis M. Avila-Barrosg, Silfredo Arrieta-Gonzalezh, Alfonso Munoz-Velasquezi, Eduardo J. Echeverry-Navarretej, Julian R. Lugo-Penak, Juan A. Ceronl, Luis E. Silva-Diazgranadosm, Hugo E. Osorio-Carmonan, Luis E. Echeverriao, Juan E. Gomez-Mesap, q, r
aDepartment of Cardiology, Clinica Medilaser, Tunja, Colombia
bDepartment of Cardiology, Centro Medico para el Corazon, Pereira, Colombia
cDepartment of Cardiology, Clinica los Rosales, Pereira, Colombia
dDepartment of Cardiology, Universidad Tecnologica de Pereira, Pereira, Colombia
eDepartment of Cardiology, Clinica Cardio VID, Medellin, Colombia
fDepartment of Cardiology, Clinica Renacer, Riohacha, Colombia
gDepartment of Internal Medicine, Clinica Riohacha, Riohacha, Colombia
hDepartment of Cardiology, Hospital Universitario de Sincelejo, Sincelejo, Colombia
iDepartment of Cardiology, Clinica Iberoamerica, Barranquilla, Colombia
jDepartment of Cardiology, Clinica Imbanaco, Cali, Colombia
kDepartment of Cardiology, Clinicos IPS, Bogota, Colombia
lDepartment of Cardiology, Hospital Departamental de Narino, Pasto, Colombia
mDepartment of Cardiology, Hospital de la Policia, Bogota, Colombia
nDepartment of Cardiology, Clinica Medilaser, Neiva, Colombia
oDepartment of Cardiology, Fundacion Cardiovascular de Colombia, Floridablanca, Colombia
pDepartment of Cardiology, Fundacion Valle del Lili, Cali, Colombia
qDepartment of Health Sciences, Universidad Icesi, Cali, Colombia
rCorresponding Author: Juan Esteban Gomez-Mesa, Department of Cardiology, Fundacion Valle del Lili, Cali, Colombia
Manuscript submitted November 20, 2024, accepted March 13, 2025, published online May 7, 2025
Short title: HF With HFpEF and HFmrEF
doi: https://doi.org/10.14740/cr2015
| Abstract | ▴Top |
Background: Heart failure with preserved or mildly reduced ejection fraction (HFpEF/HFmrEF) has differences in therapy and development when compared with HF with reduced EF (HFrEF). We aimed to describe the clinical characteristics and all-cause mortality of patients with HFpEF/HFmrEF compared to those with HFrEF from the Colombian Heart Failure Registry (RECOLFACA).
Methods: RECOLFACA included Colombian adult patients with ambulatory HF recruited from 2017 to 2019. All-cause mortality was our main outcome. We used the Kaplan-Meier method, life table, and Cox proportional hazard models to evaluate the role of the comorbidities on mortality, with a significant P-value of < 0.05. All statistical tests were two-tailed.
Results: We included 2,514 patients, and 1,139 (45.3%) had a diagnosis of HFpEF or HFmrEF. HFpEF/HFmrEF diagnosis was not significantly related to either higher or lower risk of mortality compared to an HFrEF diagnosis; however, the individual risk factors for this outcome varied between the two groups. Health-related quality of life (HRQL) was a common risk factor for both groups.
Conclusion: Although the EF classification was not a significant risk factor for mortality, patients with HFpEF/HFmrEF exhibited a unique profile of risk factors for mortality, the HRQL, highlighting the relevance of an adequate classification of the HF patients.
Keywords: Systolic blood pressure; Treatment; Comorbidities; Quality of life; Mortality
| Introduction | ▴Top |
Heart failure (HF) is a progressive clinical illness that represents a significant worldwide concern [1]. In the United States, nearly 6.2 million persons were living with HF between 2013 and 2016; however, in Latin America, the prevalence of HF was expected to be 1% (95% confidence interval (95% CI): 0.1-2.7%) [1, 2]. Although substantial advances have been made in diagnosing and treating this clinical condition, currently HF still represents one of the principal diseases with elevated rate of morbidity and mortality [3, 4].
Left ventricular ejection fraction (LVEF) has been applied as a mainstay and determines the classification and phenotyping of HF, aiding in the therapeutic approach of these patients [5]. Two to three classifications were created to improve the evaluation of the patients with HF in the daily practice: patients with preserved ejection fraction (pEF), patients with reduced ejection fraction (rEF) and, on certain patients with mildly reduced ejection fraction (mrEF) [6]. HFpEF refers to a clinical condition described by the failure of the heart to transport the necessary amount of oxygen to the tissues despite an average EF [7]. Between HFrEF and HFpEF, we can find an important group of patients with HFmrEF (EF between 41% and 49%). The prevalence of HFpEF (and also HFmrEF) has been increasing in the last decades, representing nowadays around half of the total HF cases in some regions [8]. Although there is increasing evidence of the role and implications of this entity, studies assessing the phenomenon of HFpEF in Latin America are scarce and have included small sample sizes [9-11]. Therefore, our research aimed to characterize the sociodemographic, clinical, echocardiographic, and laboratory profiles as well as all-cause mortality of patients with HFpEF/HFmrEF compared to patients with HFrEF included in the Colombian Heart Failure Registry (RECOLFACA).
| Materials and Methods | ▴Top |
Study design and population
RECOLFACA is a prospective cohort study that gathered data from 60 medical centers, heart failure clinics, and cardiology outpatient centers in Colombia. Patient recruitment began in February 2017 and ended in October 2019. All patients were ambulatory beyond the age of 18 years with a clinical diagnosis of chronic HF and a history of HF decompensation. We excluded patients with a history of heart transplant, with previous implantation of a ventricular assistance device, or those unable to comply with scheduled follow-ups. Also, patients with neurological or social limitations that might have affected adherence and pharmacological monitoring were also excluded. The registry’s extended criteria for inclusion and exclusion, along with other methodological details, were explained before [12, 13]. This research received approval from the Ethics committee of the Fundacion Valle del Lili under the act number 174-2017. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Data collection
We registered sociodemographic and clinical variables, as well as laboratory test results at baseline. The main variable of our study was all-cause mortality. Data on this outcome were collected by each HF clinic and participant institution at a ≥ 6 months follow-up. Study data were registered and managed using Research Electronic Data Capture (REDCap) electronic data capture tools [14, 15]. A list of all variables analyzed in RECOLFACA is shown in Supplementary Material 1 (cr.elmerpub.com).
HF severity was evaluated using the New York Heart Association (NYHA) classification. Those patients with LVEF ≥ 50% were categorized as HFpEF, and patients with LVEF ≤ 40% were considered HFrEF. Individuals with an EF between 41% and 49% were labeled as having HFmrEF. The comorbidities evaluated were arterial hypertension, alcoholism, smoking, type 2 diabetes mellitus, liver disease, coronary heart disease, chronic obstructive pulmonary disease (COPD), atrial fibrillation, thyroid disease, chronic kidney disease, valvular heart disease, coronary artery bypass graft (CABG), dyslipidemia, Chagas disease, and chronic kidney disease, defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2. Regarding the pharmacological treatment, we evaluated the use of triple therapy, i.e. angiotensin receptor blocker (ARB), angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor neprilysin inhibitor (ARNI), plus a mineralocorticoid receptor antagonist (MRA) and a beta-blocker, or other medicaments such as nitrates, ivabradine, anticoagulants, antiplatelets, and statins [12]. A monitoring period of 6 months was performed to evaluate pharmacological treatment, rehospitalization, and mortality.
The quality of life of the patients was assessed using the EQ-5D questionnaire, available in Spanish, which is a self-administered instrument that evaluates the health-related quality of life (HRQL) in terms of health state of an individual by using a visual analog scale (VAS) for an overall assessment. The VAS score assigns a score from 0 to 100 according to the patient’s self-assessment, with a score of 100 reflecting the best imaginable health state and a score of 0 representing the worst imaginable health state [16].
Statistical analysis
Continuous variables were described as medians and quartiles, while categorical variables were defined in terms of absolute counts, proportions, and percentages. To compare groups, categorical variables were analyzed using Pearson’s Chi-square and Fisher’s exact tests, while continuous variables were evaluated using Mann-Whitney U tests. The cumulative frequency of the mortality events was analyzed with their respective 95% CIs. We employed the Kaplan-Meier method, life table, and Cox proportional hazard models to evaluate survival analyses. A Cox’s proportional regression model encompassing both univariate and multivariate analyses was executed to identify comorbidities independently predictive of mortality, and the models’ assumptions were estimated. To calculate the effect of comorbidities on patients’ HRQL, we used a multivariate linear regression model. Every multivariate analysis was adjusted by age, sex, alcohol abuse history, smoking, and NYHA classification. Results were considered as significant with P-value of < 0.05 (two-tailed test). The Statistical Package STATA version 15 (Station College, Texas, USA) was used for the analysis.
| Results | ▴Top |
RECOLFACA registry included 2,528 patients, but only 2,514 had complete demographic and clinical variables information. The cohort median age was 69 years (Q1: 59; Q3: 78), and most of the patients were male (57.6%; n = 1,447). From the entire cohort, 1,139 patients (45.3%) were diagnosed with HFpEF or HFmrEF.
Demographic variables, comorbidities, and pharmacological treatment by EF group
Patients with HFpEF or HFmrEF were significantly older (71 vs. 68 years, P < 0.001) and were mainly woman (44.9% vs. 40.4%) when compared to those with HFrEF. Similarly, patients with HFpEF or HFmrEF presented a greater prevalence of arterial hypertension (74.9% vs. 69.6%, P = 0.003), COPD (21.1% vs. 14.6%, P < 0.001), atrial fibrillation (24.1% vs. 20.7%, P = 0.040), and valvular heart disease (19.3% vs. 15.2%, P < 0.001) compared to those with HFrEF. Additional differences were observed regarding pharmacological treatment, highlighting a more frequent utilization of ACEI/ARB (78% vs. 72%) and anticoagulants (27.9% vs. 23.6%), but a lower use of ARNI (6% vs. 13%), beta-blockers (85% vs. 89%), MRAs (41% vs. 68%), diuretics (63.1% vs. 70.8%), ivabradine (2.9% vs. 8.5%), and digoxin (7.2% vs. 12.3%) when comparing those with HFpEF or HFmrEF vs. those with HFrEF (Fig. 1, Table 1). On the other hand, patients with HFrEF exhibited a significantly lower median systolic blood pressure value (118 vs. 120 mm Hg, P < 0.001, while having a slightly upper heart rate (72 vs. 71 bpm, P = 0.044).
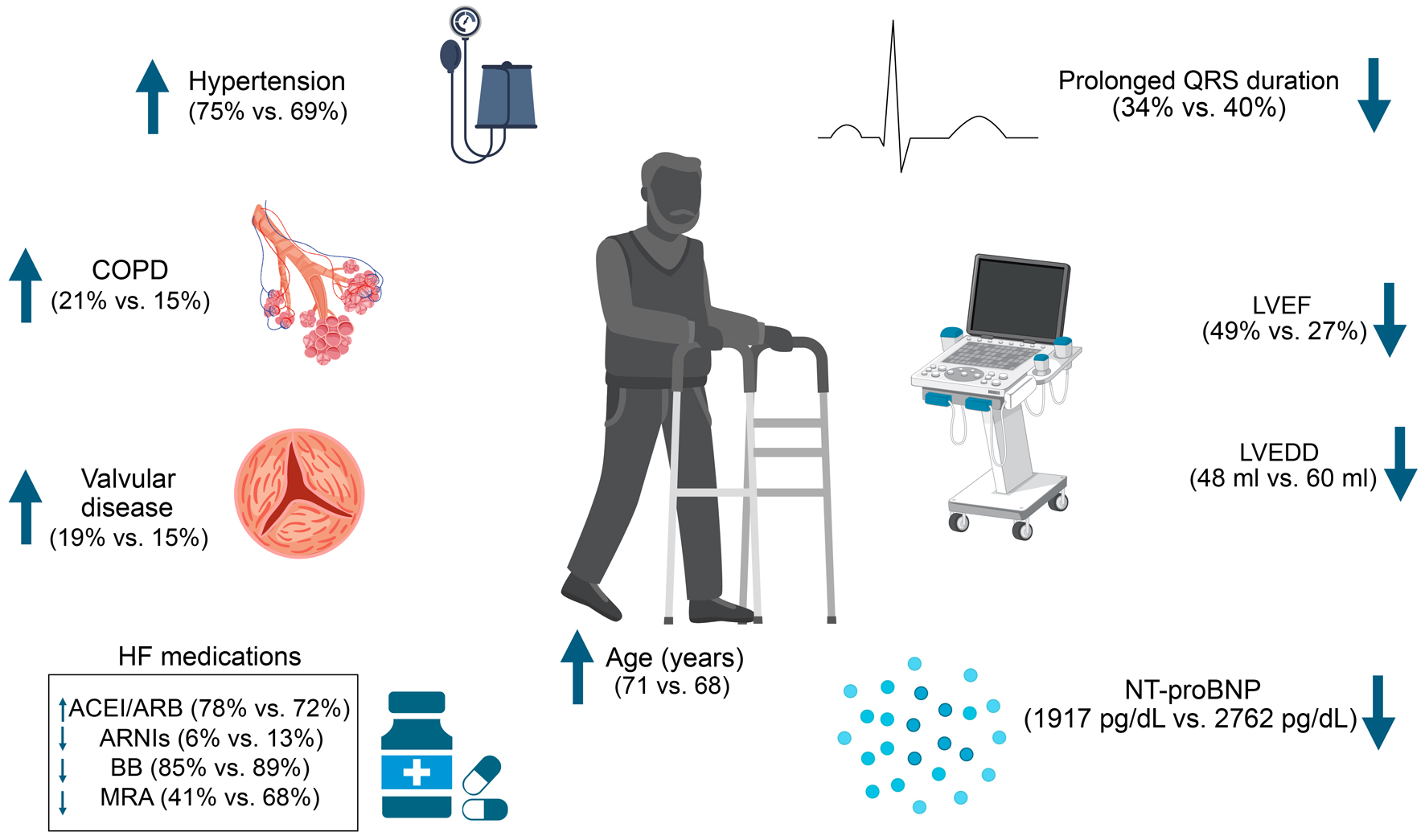 Click for large image | Figure 1. Clinical, echocardiographic, laboratory, and pharmacological differences between patients with HFpEF/HFmrEF and HFrEF. HFpEF: heart failure with preserved ejection fraction; HFmrEF: heart failure with mildly reduced ejection fraction; HFrEF: heart failure with reduced ejection fraction. |
 Click to view | Table 1. Baseline Characteristics According to the Ejection Fraction Classification |
Regarding implantable devices, patients with HFrEF presented a significantly greater rate of implantable cardioverter defibrillator (12% vs. 6.8%) and resynchronization therapy (8.9% vs. 4.5%) use when compared to those with HFpEF or HFmrEF, while presenting a lower rate of pacemaker use (bicameral: 3.2% vs. 4.7%; unicameral: 1.5% vs. 2.5%). Furthermore, patients with HFrEF presented a significantly higher LV end-diastolic diameter (60 vs. 48 mm, P < 0.001) and more frequently reported a prolonged QRS duration (40.4% vs. 34.2%). Individuals with HFpEF/HFmrEF had a lower N-terminal pro-brain natriuretic peptide (NT-proBNP) value (1917 pg/mL, P = 0.012), reporting more frequently a diagnosis of hyponatremia (16.6% vs. 11.9%, P = 0.006).
For the variable of etiology of HF, only the hypertensive, valvular, and idiopathic etiologies were significantly different between both groups (P = 0.001, P = 0.002, and P = 0.000, respectively). Both the hypertensive and valvular etiologies were higher in the HFpEF/HFmrEF group, while the idiopathic etiology was higher in the HFrEF group. The eGFR variable also showed significant differences between the groups, being higher in the HFrEF group (P = 0.006).
Impact of EF classification on mortality risk
The median follow-up was 115 days (Q1: 188; Q3: 254). During the follow-up, 170 patients (6.76%) died, representing a mortality rate of 0.30 - 1,000 person-years (95% CI: 26.2 - 35.4). Although the mortality rate per 1,000 person-years of the patients with HFrEF was greater than that of patients with HFpEF/HFmrEF (0.31 vs. 0.29, respectively), there was no statistical difference (P = 0.806). Furthermore, according to the correlation analysis between the EF classification and mortality risk, the HFpEF/HFmrEF diagnosis was not significantly related with a higher mortality risk (odds ratio (OR): 0.96; 95% CI: 0.71 - 1.30) (Fig. 2).
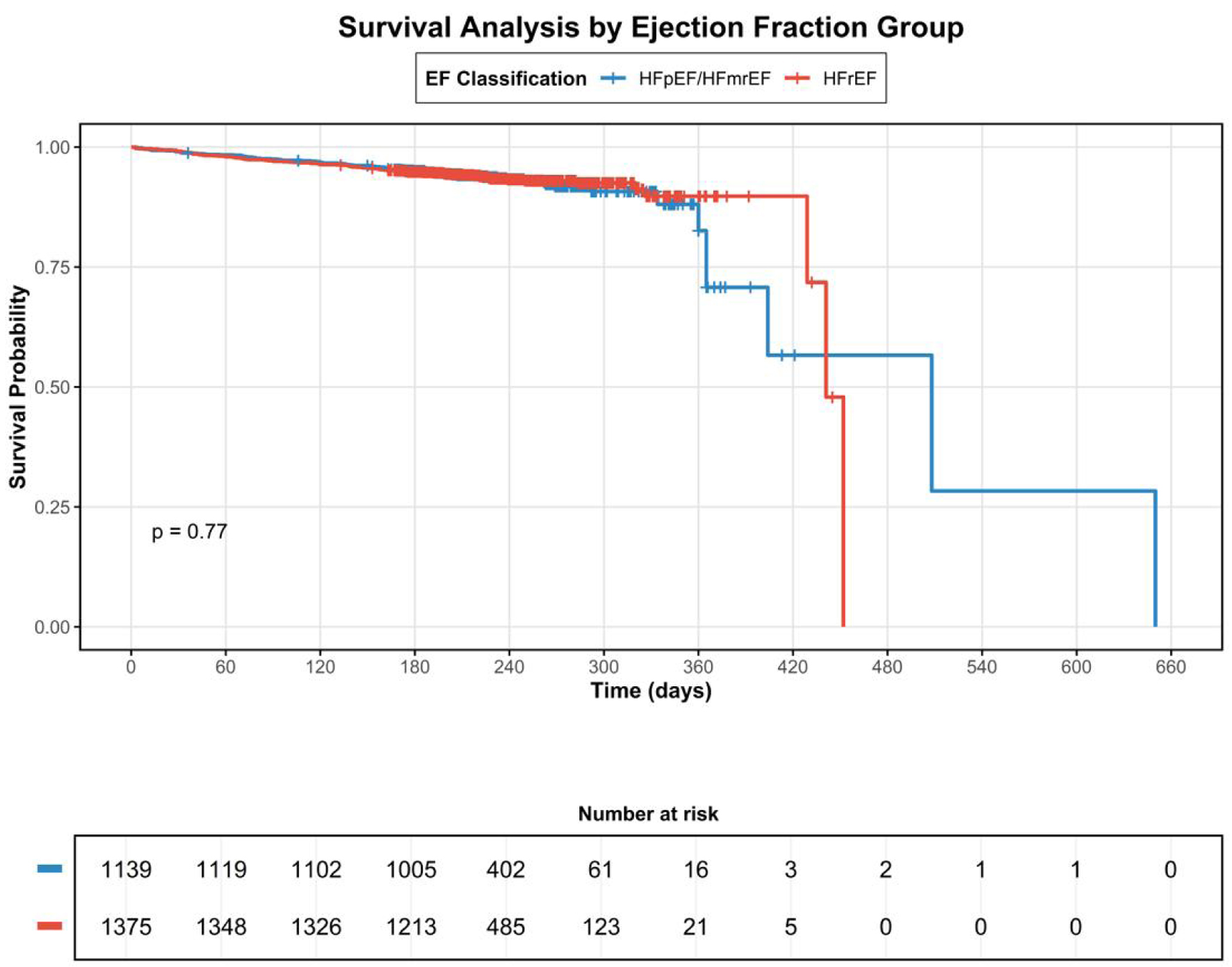 Click for large image | Figure 2. Kaplan-Meier survival curves comparing patients with HFpEF/HFmrEF versus HFrEF. HFpEF: heart failure with preserved ejection fraction; HFmrEF: heart failure with mildly reduced ejection fraction; HFrEF: heart failure with reduced ejection fraction. |
ACEI/ARB use (OR: 0.44; 95% CI: 0.26 - 0.74), hemoglobin levels (OR: 0.88; 95% CI: 0.78 - 0.99), Chagas disease diagnosis (OR: 3.86; 95% CI: 1.53 - 9.74), and HRQL score (OR: 0.97; 95% CI: 0.96 - 0.98) were individual risk factors independently related with mortality in patients with HFpEF/HFmrEF. For patients with HFrEF, the risk factors for mortality were systolic blood pressure (OR: 0.98; 95% CI: 0.96 - 0.99), nitrates use (OR: 4.03; 95% CI: 2.04 - 7.96), hyperkalemia (OR: 3.86; 95% CI: 1.53 - 9.74), the number of comorbidities (OR: 3.86; 95% CI: 1.53 - 9.74), and HRQL score (OR: 3.86; 95% CI: 1.53 - 9.74) (Table 2).
 Click to view | Table 2. Independent Predictors of All-Cause Mortality According to the Ejection Fraction Classification |
| Discussion | ▴Top |
RECOLFACA is the first Colombian registry to report epidemiology and short-term outcomes of HF with preserved and mildly reduced EF in a large cohort. This study analyzes all-cause mortality of Colombian patients with HFpEF/HFmrEF compared to those with HFrEF. Although HFpEF/HFmrEF diagnosis was not significantly related to either higher or lower risk of mortality than those with HFrEF, the individual risk factors for this outcome varied between the two groups. Only the HRQL was a common risk factor for both groups.
The observed mortality rate in our study (6.76%, or 0.30 per 1,000 person-years) is consistent with other HF registries in Latin America and reflects the severity of our study population. All included patients had a history of HF decompensation and were being followed in specialized HF clinics, representing a higher-risk cohort. Additionally, our population had a significant burden of comorbidities, including a high prevalence of arterial hypertension (72.0%), coronary heart disease (28.1%), and diabetes mellitus (24.7%), which may contribute to the observed mortality rates.
Several studies highlight that around 50% of patients with HF have HFpEF, a finding observed in our study [8]. Our results are coherent with previous reports in regards to the older age and the higher proportion of women in the HFpEF/HFmrEF group [3, 17-20]. The comorbidity profile of the patients was similar to what has been reported in other registries worldwide. However, some of the published evidence is contradictory, as some studies have reported a younger age in patients with HFpEF as to those with HFrEF [18]. These differences may appear due to relevant geographical variations, as these studies included more Afro-Colombian patients [18]. Afro-Colombians comprised only 3% of the population of RECOLFACA; however, they represent a relevant population due to their higher risk of developing arterial hypertension and HFpEF. Nevertheless, these racial differences remain understudied [21].
Beyond the baseline differences between the groups, a discussion concerning the connection between HFpEF/HFmrEF diagnosis and the risk of adverse outcomes still remains. One study observed that, despite having lower 30-day readmissions, patients with HFpEF present a similar risk of 1-year mortality after discharge related to individuals with HFrEF [18]. A similar result was detected in a study based on the Get With The Guidelines-Heart Failure registry, which assessed 39,982 patients from 254 hospitals in the USA, and reported a higher risk of cardiovascular and HF readmission rates in patients with HFpEF as to those with HFrEF. Nevertheless, a similar mortality risk concerning the two groups was observed after a follow-up of 5 years [17]. Finally, a report based on the Framingham Heart and Cardiovascular Health Study, analyzing 15,217 patients with HF, observed no significant differences in mortality rates when comparing HF subtypes (P = 0.540) [22]. Our results add to the evidence of a similar short-term scenario of patients with HFpEF (and possibly HFmrEF) and HFrEF, highlighting the relevance of other variables and conditions determining the outcomes of these patients. However, a recent study made in Poland by Rywik et al [23] showed that the risk of death was significantly higher in the group of patients with HFrEF than in those with HFpEF (61%, P < 0.001), while it was similar in both HFmrEF and HFpEF groups. These differences among the results from the studies should be further investigated.
A relevant finding of our study was the differential profile of factors associated with mortality according to the EF groups. The EQ-5D score is the only factor that patients (HFpEF/HFmrEF and HFrEF) shared. This observation is clinically relevant because it extends the understanding of the prognosis assessment in patients with HF according to the EF in Latin America, considering that the results of studies performed in other regions may not be completely generalized [24-26]. This finding contrasts with the report by Lawson et al [27] who registered that the HFpEF group had a lower HRQL when compared to the HFmrEF and HFrEF groups (P < 0.0001). In the CHARM and COACH trials, HRQL was similar in both HF groups; however, in the G-CHF study, those with HFpEF had better HRQL [27]. HRQL is an inexpensive risk predictor that could contribute to improving outcomes in patients with HF by providing crucial information for therapeutic decisions and identifying patients that need further attention to prevent hospitalizations or mortality related to HF [28].
Among factors related with mortality in patients with HFpEF/HFmrEF in our research, ACEI/ARB use was corresponding with a significantly lower risk of this outcome, highlighting the benefit of HF therapy in these patients, as several studies have indicated [29, 30]. On the other hand, a higher hemoglobin value was also associated with a significantly lower mortality risk in these patients. Anemia has been recognized as a strong predictor of hospital readmission [31]; however, a direct relationship between this condition and mortality in this context is still debated, as it has been found that anemia may be a severity marker in congestive patients but not a predictor of death [32, 33]. Interestingly, Chagas disease diagnosis was related with a significantly higher mortality risk only in patients with HFpEF/HFmrEF group. Chronic Chagas cardiomyopathy is a particular form of myocardial involvement secondary to Trypanosoma cruzi infection of the myocardium and Chagas disease [34, 35]. It is described with high burden of arrhythmias, the progress of ventricular aneurysms, and a gradual worsening of myocardial function despite optimal medical therapy [36]. A potential hypothesis to this observed association could be related to the arrhythmogenic nature of this cardiomyopathy, leading to a very high incidence of electrical complications, which has positioned sudden cardiac death among the primary cause of mortality in this population [37]. While the arrhythmic burden is inversely correlated with the EF, patients without HFrEF can develop severe conduction system involvements, resulting in a significant risk of sudden cardiac death [38, 39]. The prognostic implications of this finding suggest the need for specific therapeutic approaches in this subgroup, including early screening strategies in endemic areas, systematic monitoring for arrhythmic complications, and potentially earlier consideration of device therapy [40]. Furthermore, the timing of antiparasitic therapy in the context of established HF requires careful evaluation, as it has not shown benefits on clinical outcomes in this setting [41]. These considerations are particularly relevant in Latin America, where Chagas disease continues to represent a significant public health challenge. Future research should focus on developing tailored therapeutic strategies for this high-risk population, particularly considering their unique pathophysiological characteristics and response to standard HF therapies [42, 43].
Regarding the systolic blood pressure as a risk factor for mortality found in the HFrEF group, similar results have been previously reported by Lang et al [44] in a retrospective cohort of 1,279 Chinese patients with HF complicated by hypertension. The authors found that in HFrEF patients, systolic blood pressure < 130 mm Hg was associated with an increased risk of all-cause and cardiovascular death (HR: 2.53, 95% CI: 1.23 - 5.20, P = 0.011). Likewise, Arundel et al [45] analyzed 30-day and 1-year risk of mortality in 10,356 participants with HFrEF, finding that a systolic blood pressure between 110 and 129 mm Hg was associated with a higher risk of mortality. On the other hand, the effect on mortality and rehospitalization of the use of nitrates in patients with HF has also been studied previously by Ural et al [46] who found no beneficial effects and even a tendency to increase risk of mortality. Moreover, hyperkalemia as a mortality risk factor in patients with HFrEF has not been well studied; however, Lopez-Lopez et al [47] found that potassium levels in the normal-high range (5 - 5.5 mEq/L) in patients with HFrEF might be safe and not associated with increased mortality.
While our study showed similar mortality rates between groups, the characterization of patients by EF remains clinically relevant as we found distinct risk factor profiles for mortality between groups, suggesting different pathophysiological mechanisms that may require targeted interventions. Moreover, our findings showed significant differences in medication utilization patterns between groups (e.g., higher use of ACEI/ARB in HFpEF/HFmrEF vs. higher use of beta-blockers and MRAs in HFrEF). This characterization helps identify specific comorbidity patterns (higher prevalence of COPD, atrial fibrillation, and valvular heart disease in HFpEF/HFmrEF) that may require specific management strategies.
Study limitations
The patients were identified as having HFrEF or HFpEF/HFmrEF using the information from only one capacity of the EF, limiting the possibility of assessing the variations of this variable across time, which could even reclassify the patients in different groups from the one assigned during the basal assessment. Second, the current data in the RECOLFACA registry did not cover information concerning pharmacological and non-pharmacological therapy regarding resisted comorbidities, which imposed constraints on the incorporation of comorbidities in the analyses of risk factors. The availability of data regarding the severity and duration of comorbidities, and optimal guideline therapy was lacking, which restricted a comprehensive evaluation of the influence of these conditions. Although the study population analyzed was not as large as other studies that show a difference in mortality, our study does include the largest population for an analysis with Colombian population. The short follow-up time represented a significant limitation in assessing long-term clinical outcomes in our HF population. While this timeframe allowed us to analyze acute and subacute events, the natural history and progression of HF typically extends beyond this period, potentially limiting our ability to capture the full spectrum of clinically relevant outcomes. Also, as the cause of death was not included in this study, the association between preserved/mildly reduced EF and mortality must be carefully interpreted. We suggest that future studies include this variable to draw more precise conclusions. Finally, we did not evaluate both populations, HFmrEF and HFpEF, separately, comparing each group against HFrEF.
Conclusions
HFpEF and HFmrEF represent two prevalent conditions with a differential clinical and prognostic profile compared to HFrEF. Despite having similar mortality rates, patients with HFpEF/HFmrEF show a characteristic pattern of risk factors for this fatal outcome compared with patients with HFrEF, highlighting the importance of an optimal classification of the HF patients. The results found in RECOLFACA, although similar to the findings in other countries, are of value to the current knowledge in HF as they come from a registry that included only Colombian population. However, more extensive population-based analyses are necessary to corroborate these findings and thoroughly assess the profile of HFmrEF and HFpEF in the Latin American region.
| Supplementary Material | ▴Top |
Suppl 1. Variables analyzed in RECOLFACA.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they do not have any competing interests.
Informed Consent
Not applicable.
Author Contributions
JEGM contributed to the conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, supervision, validation, visualization, writing - original draft, and writing - review and editing. CS and LEE contributed to the conceptualization, investigation, methodology, supervision, writing - original draft, and writing - review and editing. ART contributed to the investigation, supervision, writing - original draft, and writing - review and editing. LNMR contributed to the investigation, writing - original draft, and writing - review and editing. RP, LMAB, SAG, AMV, EJEN, JRLP, JAC, LESD, and HEOC contributed to the investigation and writing - review and editing. All authors critically revised and approved the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article and its supplementary information file.
Abbreviations
ACC: American College of Cardiology; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; ARNI: angiotensin receptor neprilysin inhibitor; AF: atrial fibrillation; AHA: American Heart Association; CABG: coronary artery bypass graft; COPD: chronic obstructive pulmonary disease; CRT: cardiac resynchronization therapy; eGFR: estimated glomerular filtration rate; HF: heart failure; HFmrEF: heart failure with mildly reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; HRQL: health-related quality of life; ICD: implantable cardioverter defibrillator; LVEF: left ventricle ejection fraction; MRA: mineralocorticoid receptor antagonist; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; QoL: quality of life; RECOLFACA: Colombian Heart Failure Registry; REDCap: Research Electronic Data Capture; VAS: visual analog scale
| References | ▴Top |
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139-e596.
doi pubmed - Ciapponi A, Alcaraz A, Calderon M, Matta MG, Chaparro M, Soto N, Bardach A. Burden of heart failure in latin america: a systematic review and meta-analysis. Rev Esp Cardiol (Engl Ed). 2016;69(11):1051-1060.
doi pubmed - Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, Parissis J, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19(12):1574-1585.
doi pubmed - Chaudhry SP, Stewart GC. Advanced heart failure: prevalence, natural history, and prognosis. Heart Fail Clin. 2016;12(3):323-333.
doi pubmed - Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129-2200.
doi pubmed - Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer RA, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(10):1169-1186.
doi pubmed - Cleland JG, Pellicori P. Defining diastolic heart failure and identifying effective therapies. JAMA. 2013;309(8):825-826.
doi pubmed - Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591-602.
doi pubmed - Moutinho MA, Colucci FA, Alcoforado V, Tavares LR, Rachid MB, Rosa ML, Ribeiro ML, et al. Heart failure with preserved ejection fraction and systolic dysfunction in the community. Arq Bras Cardiol. 2008;90(2):132-137.
doi pubmed - Petersen LC, Danzmann LC, Bartholomay E, Bodanese LC, Donay BG, Magedanz EH, Azevedo AV, et al. Survival of patients with acute heart failure and mid-range ejection fraction in a developing country - a cohort study in South Brazil. Arq Bras Cardiol. 2021;116(1):14-23.
doi pubmed - Albuquerque DC, Neto JD, Bacal F, Rohde LE, Bernardez-Pereira S, Berwanger O, Almeida DR, et al. I Brazilian registry of heart failure - clinical aspects, care quality and hospitalization outcomes. Arq Bras Cardiol. 2015;104(6):433-442.
doi pubmed - Gomez-Mesa JE, Saldarriaga-Giraldo CI, Echeverria LE, et al. Registro colombiano de falla cardiaca (RECOLFACA): resultados. Rev Colomb Cardiol. 2021;28:334-342.
- Gomez-Mesa JE, Saldarriaga CI, Echeverria LE, et al. Colombian heart failure registry (RECOLFACA): methodology and preliminary data. Rev Colomb Cardiol. 2021;28:217-230.
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
doi pubmed - Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
doi pubmed - Herdman M, Badia X, Berra S. [EuroQol-5D: a simple alternative for measuring health-related quality of life in primary care]. Aten Primaria. 2001;28(6):425-430.
doi pubmed - Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70(20):2476-2486.
doi pubmed - Quiroz R, Doros G, Shaw P, Liang CS, Gauthier DF, Sam F. Comparison of characteristics and outcomes of patients with heart failure preserved ejection fraction versus reduced left ventricular ejection fraction in an urban cohort. Am J Cardiol. 2014;113(4):691-696.
doi pubmed - Lejeune S, Roy C, Slimani A, Pasquet A, Vancraeynest D, Beauloye C, Vanoverschelde JL, et al. Heart failure with preserved ejection fraction in Belgium: characteristics and outcome of a real-life cohort. Acta Cardiol. 2021;76(7):697-706.
doi pubmed - Ergatoudes C, Schaufelberger M, Andersson B, Pivodic A, Dahlstrom U, Fu M. Non-cardiac comorbidities and mortality in patients with heart failure with reduced vs. preserved ejection fraction: a study using the Swedish Heart Failure Registry. Clin Res Cardiol. 2019;108(9):1025-1033.
doi pubmed - Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138-2145.
doi pubmed - Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018;6(8):678-685.
doi pubmed - Rywik TM, Wisniewska A, Ceglowska U, Drohomirecka A, Topor-Madry R, Lazarczyk H, Polaska P, et al. Heart failure with reduced, mildly reduced, and preserved ejection fraction: outcomes and predictors of prognosis. Pol Arch Intern Med. 2023;133(12):16522.
doi pubmed - Glynn PA, Molsberry R, Harrington K, Shah NS, Petito LC, Yancy CW, Carnethon MR, et al. Geographic variation in trends and disparities in heart failure mortality in the United States, 1999 to 2017. J Am Heart Assoc. 2021;10(9):e020541.
doi pubmed - Liu L, Yin X, Chen M, Jia H, Eisen HJ, Hofman A. Geographic variation in heart failure mortality and its association with hypertension, diabetes, and behavioral-related risk factors in 1,723 counties of the United States. Front Public Health. 2018;6:132.
doi pubmed - Kristensen SL, Martinez F, Jhund PS, Arango JL, Belohlavek J, Boytsov S, Cabrera W, et al. Geographic variations in the PARADIGM-HF heart failure trial. Eur Heart J. 2016;37(41):3167-3174.
doi pubmed - Lawson CA, Benson L, Squire I, Zaccardi F, Ali M, Hand S, Kadam U, et al. Changing health related quality of life and outcomes in heart failure by age, sex and subtype. EClinicalMedicine. 2023;64:102217.
doi pubmed - Johansson I, Joseph P, Balasubramanian K, McMurray JJV, Lund LH, Ezekowitz JA, Kamath D, et al. Health-related quality of life and mortality in heart failure: the global congestive heart failure study of 23 000 patients from 40 countries. Circulation. 2021;143(22):2129-2142.
doi pubmed - Alzahrani T, Tiu J, Panjrath G, Solomon A. The effect of angiotensin-converting enzyme inhibitors on clinical outcomes in patients with ischemic cardiomyopathy and midrange ejection fraction: a post hoc subgroup analysis from the PEACE trial. Ther Adv Cardiovasc Dis. 2018;12(12):351-359.
doi pubmed - Khan MS, Fonarow GC, Khan H, Greene SJ, Anker SD, Gheorghiade M, Butler J. Renin-angiotensin blockade in heart failure with preserved ejection fraction: a systematic review and meta-analysis. ESC Heart Fail. 2017;4(4):402-408.
doi pubmed - Jain A, Arora S, Patel V, Raval M, Modi K, Arora N, Desai R, et al. Etiologies and predictors of 30-day readmission in heart failure: an updated analysis. Int J Heart Fail. 2023;5(3):159-168.
doi pubmed - Scicchitano P, Iacoviello M, Massari A, De Palo M, Potenza A, Landriscina R, Abruzzese S, et al. Anaemia and congestion in heart failure: correlations and prognostic role. Biomedicines. 2023;11(3):972.
doi pubmed - Garcia-Torrecillas JM, Lea-Pereira MC, Alonso-Morillejo E, Moreno-Millan E, de la Fuente-Arias J. Structural model of biomedical and contextual factors predicting in-hospital mortality due to heart failure. J Pers Med. 2023;13(6):995.
doi pubmed - Bonney KM, Luthringer DJ, Kim SA, Garg NJ, Engman DM. Pathology and pathogenesis of Chagas heart disease. Annu Rev Pathol. 2019;14:421-447.
doi pubmed - Echeverria LE, Morillo CA. American Trypanosomiasis (Chagas Disease). Infect Dis Clin North Am. 2019;33(1):119-134.
doi pubmed - Rojas LZ, Glisic M, Pletsch-Borba L, Echeverria LE, Bramer WM, Bano A, Stringa N, et al. Electrocardiographic abnormalities in Chagas disease in the general population: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(6):e0006567.
doi pubmed - Neeki MM, Park M, Sandhu K, Seiler K, Toy J, Rabiei M, Adigoupula S. Chagas disease-induced sudden cardiac arrest. Clin Pract Cases Emerg Med. 2017;1(4):354-358.
doi pubmed - Stein C, Migliavaca CB, Colpani V, da Rosa PR, Sganzerla D, Giordani NE, Miguel S, et al. Amiodarone for arrhythmia in patients with Chagas disease: A systematic review and individual patient data meta-analysis. PLoS Negl Trop Dis. 2018;12(8):e0006742.
doi pubmed - Keegan R, Yeung C, Baranchuk A. Sudden cardiac death risk stratification and prevention in chagas disease: a non-systematic review of the literature. Arrhythm Electrophysiol Rev. 2020;9(4):175-181.
doi pubmed - Nunes MCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverria LE, Dutra WO, et al. Chagas cardiomyopathy: an update of current clinical knowledge and management: a scientific statement from the American Heart Association. Circulation. 2018;138(12):e169-e209.
doi pubmed - Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A, Jr., Rosas F, Villena E, et al. Randomized trial of benznidazole for chronic Chagas' cardiomyopathy. N Engl J Med. 2015;373(14):1295-1306.
doi pubmed - Echeverria LE, Serrano-Garcia AY, Rojas LZ, Berrios-Barcenas EA, Gomez-Mesa JE, Gomez-Ochoa SA. Mechanisms behind the high mortality rate in chronic Chagas cardiomyopathy: Unmasking a three-headed monster. Eur J Heart Fail. 2024;26(12):2502-2514.
doi pubmed - Llerena-Velastegui J, Lopez-Usina A, Mantilla-Cisneros C. Advances in the understanding and treatment of chronic chagas cardiomyopathy. Cardiol Res. 2024;15(5):340-349.
doi pubmed - Lang X, Peng C, Zhang Y, Gao R, Zhao B, Li Y, Zhang Y. Correlation between systolic blood pressure and mortality in heart failure patients with hypertension. J Hypertens. 2024;42(6):1048-1056.
doi pubmed - Arundel C, Lam PH, Gill GS, Patel S, Panjrath G, Faselis C, White M, et al. Systolic blood pressure and outcomes in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73(24):3054-3063.
doi pubmed - Ural D, Kandemir AS, Karauzum K, Baydemir C, Karauzum IY, Bozyel S, Kozdag G, et al. Effect of oral nitrates on all-cause mortality and hospitalization in heart failure patients with reduced ejection fraction: a propensity-matched analysis. J Card Fail. 2017;23(4):286-292.
doi pubmed - Lopez-Lopez A, Franco-Gutierrez R, Perez-Perez AJ, Regueiro-Abel M, Elices-Teja J, Abou-Jokh-Casas C, Gonzalez-Juanatey C. Impact of hyperkalemia in heart failure and reduced ejection fraction: a retrospective study. J Clin Med. 2023;12(10):3595.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.