| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 1, February 2025, pages 33-43
Atrial Fibrillation Recurrence Post-Ablation Across Heart Failure Categories: A Systematic Review and Meta-analysis
Carl Hashema, h, Jacob Josephb, c, d, Scott Kinlayd, e, f, g, Adelqui O. Peraltad, e, f, Peter S. Hoffmeisterd, e, f, Matthew F. Yuyund, e, f
aDepartment of Medicine, New York University Grossman School of Medicine, New York, NY, USA
bDivision of Cardiology, VA Providence Healthcare System, Providence, RI, USA
cDivision of Cardiology, Brown University Warren Alpert School of Medicine, Providence, RI, USA
dDivision of Cardiology, VA Boston Healthcare System, Boston, MA, USA
eDivision of Cardiology, Boston University Chobanian and Avedisian School of Medicine, Boston, MA, USA
fDivision of Cardiology, Harvard Medical School, Boston, MA, USA
gDivision of Cardiology, Brigham and Women’s Hospital, Boston, MA, USA
hCorresponding Author: Carl Hashem, Department of Medicine, New York University Grossman School of Medicine, New York, NY 10016, USA
Manuscript submitted November 26, 2024, accepted January 9, 2025, published online January 21, 2025
Short title: AF Recurrence Post-Ablation Across HF Categories
doi: https://doi.org/10.14740/cr2020
| Abstract | ▴Top |
Background: Previous studies have provided evidence of reduced recurrence of atrial fibrillation (AF), all-cause mortality, and heart failure (HF) hospitalizations after catheter ablation (CA) in both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). Aggregate data comparing the efficacy of AF ablation and clinical endpoints in HF with mildly reduced ejection fraction (HFmrEF) to HFrEF and HFpEF are lacking.
Methods: We conducted a systematic review and meta-analysis aimed at determining any differences in AF recurrence rate, all-cause mortality, and HF hospitalizations among patients with HFrEF, HFmrEF, and HFpEF who underwent AF ablation. A systematic search of PubMed/MEDLINE, Embase, and Cochrane Library databases was performed until October 31, 2023.
Results: A total of seven studies comprising 3,795 patients were retained: HFrEF 1,281 (33.8%), HFmrEF 870 (22.9%), and HFpEF 1,644 (43.3%). After median follow-up of 24 months, there was no significant difference in rate of AF recurrence between the three HF categories: HFrEF 40% (30-49%), HFmrEF 35% (28-43%); and HFpEF 35% (25-45%). Only two studies which included outcomes in the three HF categories were identified. Pooled hazard ratio (HR) of all-cause mortality and HF hospitalization combined after ablation or other rhythm control compared to other conservative management were: HFrEF 0.77 (0.63 - 0.94); HFmrEF 0.81 (0.55 - 1.20); and HFpEF 0.74 (0.55 - 1.00).
Conclusions: CA has similar efficacy in the long-term resolution of AF among patients with HFrEF, HFmrEF, and HFpEF. Further studies are needed to provide a robust analysis on the potential impact of CA on all-cause mortality.
Keywords: Atrial fibrillation; Ablation; Heart failure with preserved ejection fraction; Heart failure with mildly reduced ejection fraction; Heart failure with reduced ejection fraction
| Introduction | ▴Top |
Atrial fibrillation (AF) and heart failure (HF) are among the most commonly encountered cardiac disorders occurring with increasing incidence and prevalence. Both conditions are strongly associated with one another and are significant independent causes of cardiovascular morbidity and mortality [1, 2]. The presence of AF has been found to accentuate the risk of all-cause mortality and HF hospitalizations among HF subgroups [3-6].
Catheter ablation (CA) as a treatment for AF has become increasingly common, with evidence of decreased atrial arrhythmia, along with decreased mortality and HF hospitalizations when compared to management with antiarrhythmic drugs (AADs) in the general population [7]. Similarly, the management of CA for patients with concomitant HF and AF has been of particular interest in recent years, with several studies and meta-analyses demonstrating lowered incidence of AF recurrence, mortality, and HF hospitalizations among patients with HF with reduced ejection fraction (HFrEF) [8-10]. Among patients with HF with preserved ejection fraction (HFpEF), CA has similarly been associated with a reduction in atrial recurrence, mortality, and HF hospitalizations when compared to standard medical therapy [11, 12]. However, a reduction in cardiovascular outcomes such as mortality and HF hospitalizations are not universally seen with CA in HFpEF [13, 14]. When compared directly, AF ablation in HFrEF appears to have similar rates of atrial recurrence and heart hospitalizations to AF ablation in HFpEF [15]. Data regarding mortality are conflicting as one meta-analysis suggests a higher rate of mortality in HFrEF compared to HFpEF [16].
Contemporary classification of HF has evolved in recent years with the formal recognition of HF with mildly reduced ejection fraction (HFmrEF) as a distinct phenotype. HFmrEF, defined as HF with left ventricular ejection fraction (LVEF) in the range of 41-49%, represents a particular subset of HF which had been commonly excluded from earlier clinical trials [17]. Prior to this definition, studies employed inconsistent cut-offs for HFrEF and HFpEF, creating an ill-defined middle range, and causing confusion when implementing guidelines for therapeutic recommendations in the two former groups. Following the formal recognition of HFmrEF, the number of studies evaluating this subtype has increased rapidly.
In this systematic review and meta-analysis, we aim to evaluate and compare the efficacy of CA as management for AF in patients with HFrEF, HFmrEF, and HFpEF with respect to AF recurrence, and combined all-cause mortality and HF hospitalizations.
| Materials and Methods | ▴Top |
Prior to data collection, this study was registered with the international prospective register of systematic reviews (PROSPERO) with the registration number CRD42023404929. The manuscript is presented according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) requirements.
Search strategy
All relevant English language studies restricted to human adults published from inception until October 31, 2023, were systematically searched from PubMed/MEDLINE, Embase, and Cochrane Library databases. The search terms used were as follows: “(atrial fibrillation OR atrial flutter OR atrial arrhythmia) AND (ablation OR pulmonary vein isolation OR catheter ablation OR cryoablation OR radiofrequency ablation OR rhythm control OR antiarrhythmic medications OR antiarrhythmic drugs) AND (heart failure with reduced ejection fraction OR HFrEF OR heart failure with preserved ejection fraction OR HFpEF OR heart failure with mid-range ejection fraction OR heart failure with mildly reduced ejection fraction OR HFmrEF)”.
Inclusion criteria
Studies reporting on atrial arrhythmia (AF or atrial tachycardia or atrial flutter) recurrence after an ablation across the three HF categories were included. The HF categories based on LVEF were defined as: HF with reduced LVEF - HFrEF (LVEF ≤ 40%); HF with mildly reduced LVEF - HFmrEF (LVEF 41-49%); and HF with preserved LVEF - HFpEF (LVEF ≥ 50%). As a prerequisite, included studies had to have all the three categories of HF based on LVEF.
Exclusion criteria
Excluded were non-English language studies lacking an English-translated version. Also excluded were studies that did not report on findings across all the three categories of HF and studies that did not report on any of the outcomes or reported outcomes in combinations that did not satisfy the objectives of this meta-analysis. The CONSORT diagram is presented in Figure 1.
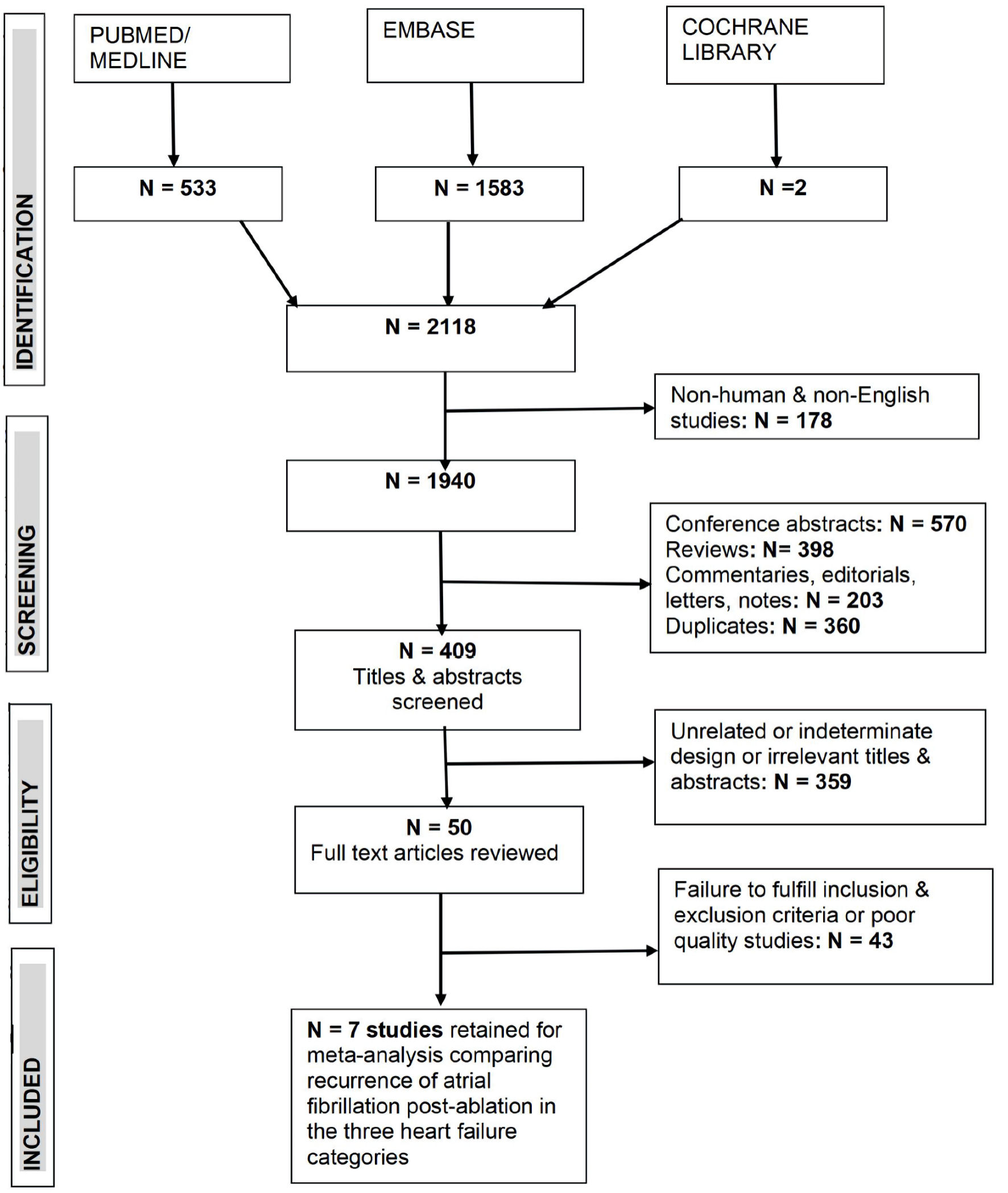 Click for large image | Figure 1. CONSORT diagram of literature search and identification of relevant studies all three heart failure categories based on left ventricular ejection fraction and atrial fibrillation. |
Outcomes
The main outcome was recurrence rate of AF during follow-up across the three categories of HF (HFrEF, HFmrEF, and HFpEF). Secondary outcomes were the impact of CA or other rhythm control strategy on combined all-cause mortality and HF hospitalization.
Quality assessment of studies
The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analyses was used for quality assessment [18]. We categorized the studies according to NOS as follows: 0 - 3 = poor quality, 4 - 7 = fair quality, 8 - 9 = good quality.
Data extraction
Two authors (CH, MFY) extracted the data independently using standardized forms containing pre-defined demographic and clinical information including AF, HF categories, outcomes, duration of follow-up, and quality assessment. Discrepancies were resolved by consensus.
Statistical analysis
All analyses were performed using the STATA 18 software package (Stata Corp, Texas). Study characteristics such as duration of follow-up, mean age, etc., were combined using study size as analytical weights to yield single pooled estimates (weighted average). The method for pooling study specific estimates was a priori determined to be random-effects model (DerSimonian-Laird) as some degree of heterogeneity was anticipated. The rate of AF recurrence in each HF phenotype group was pooled. Hazard ratios (HRs) of all-cause mortality and HF hospitalization combined were also pooled. The statistical significance of the pooled relative risk was examined by the Z-test (statistical test of the null hypothesis). A two-sided P value < 0.05 was considered statistically significant. Results are presented as pooled estimates and 95% confidence intervals (95% CIs).
The magnitude of heterogeneity across studies was assessed using the I2 statistic, where I2 = ((Q- df)/Q) × 100%, with Q being the Cochran’s heterogeneity statistic and df its degrees of freedom [19]. The I2 statistic describes the percentage variability in effect estimates that is due to true between study heterogeneity (difference) rather than sampling error (chance). When I2 was < 25%, heterogeneity was considered absent; when I2 was 25-50%, heterogeneity was considered low; when I2 was 50-75%, heterogeneity was considered moderate; and when I2 was > 75%, heterogeneity was considered high [19]. Publication bias was assessed by visual scrutiny of a funnel plot of study-specific estimates by the study standard errors. When funnel plot asymmetry was observed, a contour-enhanced funnel plot was fitted to determine whether it was attributed to publication bias [20].
The Institutional Review Board approval is not applicable to this study. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects, as well as with the Helsinki Declaration. The study exclusively utilized data that were previously published and publicly available. As such, no new data collection was undertaken, and no direct interaction with human participants occurred.
| Results | ▴Top |
The initial search identified 2,118 citations from PubMed, Embase, and Cochrane Library. From these, only seven studies [18, 19, 21-25] with 3,795 patients, which included all the three HF categories that reported AF recurrence after CA, were retained after application of inclusion criteria, exclusion criteria, and quality assessment for the systematic review and meta-analysis. Of these 3,795 patients, 1,281 (33.8%), were classified as HFrEF, 870 (22.9%) were HFmrEF, and 1,644 (43.3%) were HFpEF (Table 1, Fig. 1) [18, 19, 21-25].
 Click to view | Table 1. Baseline Characteristic of Studies Comparing Atrial Fibrillation Recurrence Post-Ablation Across the Three Standard Categories of Heart Failure |
Baseline characteristics
Baseline characteristics of the included studies are shown in Table 1 [18, 19, 21-25]. The pooled proportion or mean of variables between the HF categories weighted by sample size were as follows: men HFrEF (80.6%), HFmrEF (71.5%), HFpEF (63.0%); age HFrEF (66.0 years), HFmrEF (67.0 years), HFpEF (67.0 years); diabetes HFrEF (25.6%), HFmrEF (24.2%), HFpEF (20.5%); coronary artery disease (CAD) HFrEF (31.6%), HFmrEF (30.9%), HFpEF (33.1%); hypertension HFrEF (66.1%), HFmrEF (69.8%), HFpEF (74.0%); LVEF HFrEF (32.0%), HFmrEF (44.5%), HFpEF (61.2%); anti-arrhythmic medication HFrEF (34.4%), HFmrEF (29.9%), HFpEF (34.5%); chronic kidney disease (CKD) HFrEF (20.4%), HFmrEF (12.5%), HFpEF (12.3%); stroke/transient ischemic attack (TIA) HFrEF (8.9%), HFmrEF (6.7%), HFpEF (8.7%). Only LVEF and CKD showed significant differences between the three HF groups, with worse profiles in HFrEF. The majority of the included studies recruited ablation-naive patients undergoing a first-time procedure. One study [19] did not comment on prior ablation. Eitel et al [22] reported the following proportion of patients who underwent a first-time procedure: HFrEF (89.4%), HFmrEF (82.7%), HFpEF (80.2%).
AF recurrence
Median duration of follow-up after AF ablation was 24 months (interquartile range 12 - 31.2 months). As shown in Table 2 [18, 19, 21-25]. and the forest plot (Fig. 2), there was no significant difference in the rate of AF occurrence between the three HF categories: HFmrEF 35% (95% CI: 28-43%); HFpEF 35% (25-45%); and HFrEF 40% (30-49%).
 Click to view | Table 2. Atrial fibrillation Recurrence During Follow-Up |
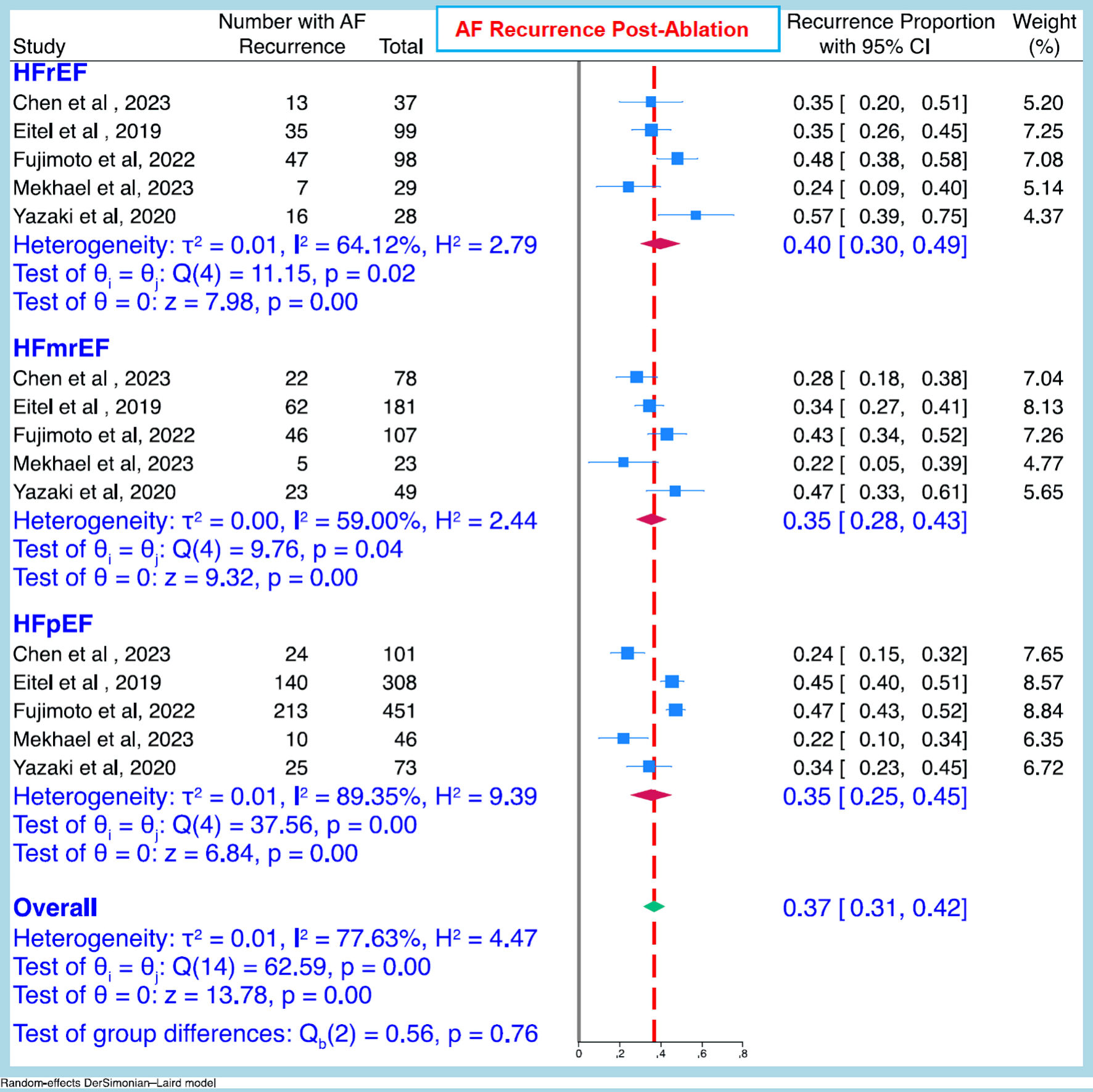 Click for large image | Figure 2. Pooled atrial fibrillation recurrence rate (95% CI) after ablation between the three heart failure categories. CI: confidence interval; AF: atrial fibrillation; HFrEF: heart failure with reduced ejection fraction; HFmrEF: heart with mildly reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction. |
Comparison of all-cause mortality and HF hospitalization post-ablation or rhythm control versus other conservative management
Only two studies [18, 25] which included outcomes in the three HF categories were identified. The pooled HR and (95% CI) of all-cause mortality and HF hospitalization combined after CA or other rhythm control compared to other conservative management were: all patients HR 0.77 (95% CI: 0.66 - 0.90); HFrEF patients HR 0.77 (95% CI: 0.63 - 0.94); HFpEF 0.74 (0.55 - 1.00), and HFmrEF 0.81 (0.55 - 1.20) (Fig. 3).
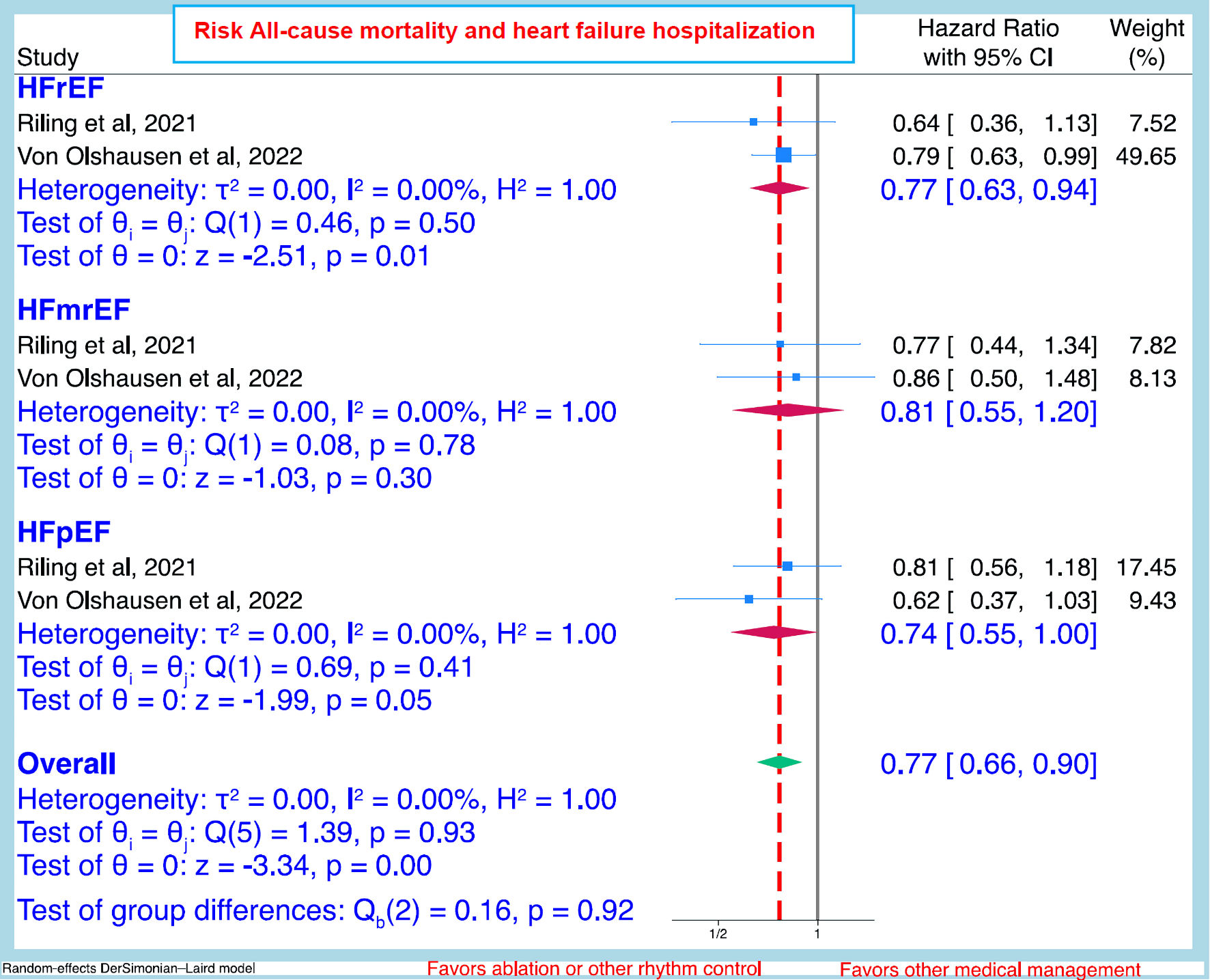 Click for large image | Figure 3. Pooled hazard ratio (95% CI) of all-cause mortality and heart failure hospitalization after ablation or other rhythm control compared to other conservative management between the heart failure categories. CI: confidence interval; HFrEF: heart failure with reduced ejection fraction; HFmrEF: heart with mildly reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction. |
Two studies [22, 23], which included only patients who all underwent AF ablation without a control group of medical therapy, had inconsistent findings of all-cause mortality and HF hospitalization rates between the three HF phenotypes. For example, Fujimoto et al [23] depicted significantly higher incidence rate of a composite of all-cause mortality and HF hospitalization in HFrEF (32.7%) compared to HFmrEF (11.7%), and HFpEF (11.6%), P < 0.001 (all-cause mortality alone HFrEF (9.5%), HFmrEF (3.2%), HFpEF (3.9%), P = 0.009; HF hospitalization alone HFrEF (27.3%), HFmrEF (6.6%), HFpEF (7.1%), P < 0.001; and cardiovascular mortality HFrEF (4.4%), HFmrEF (1.2%), HFpEF (14%), P = 0.038) [23]. For Eitel et al [22], there was no significant difference in mortality rate between the three HF groups after 12 months of follow-up: HFrEF (1.1%), HFmrEF (0%), HFpEF (1.3%), P = 0.31.
Publication bias
The funnel plot and contour-enhanced funnel plot of included studies of AF recurrence across the HF phenotypes are shown in Figure 4. There was no evidence of publication bias or small study effects with Egger’s test P value of 0.4175.
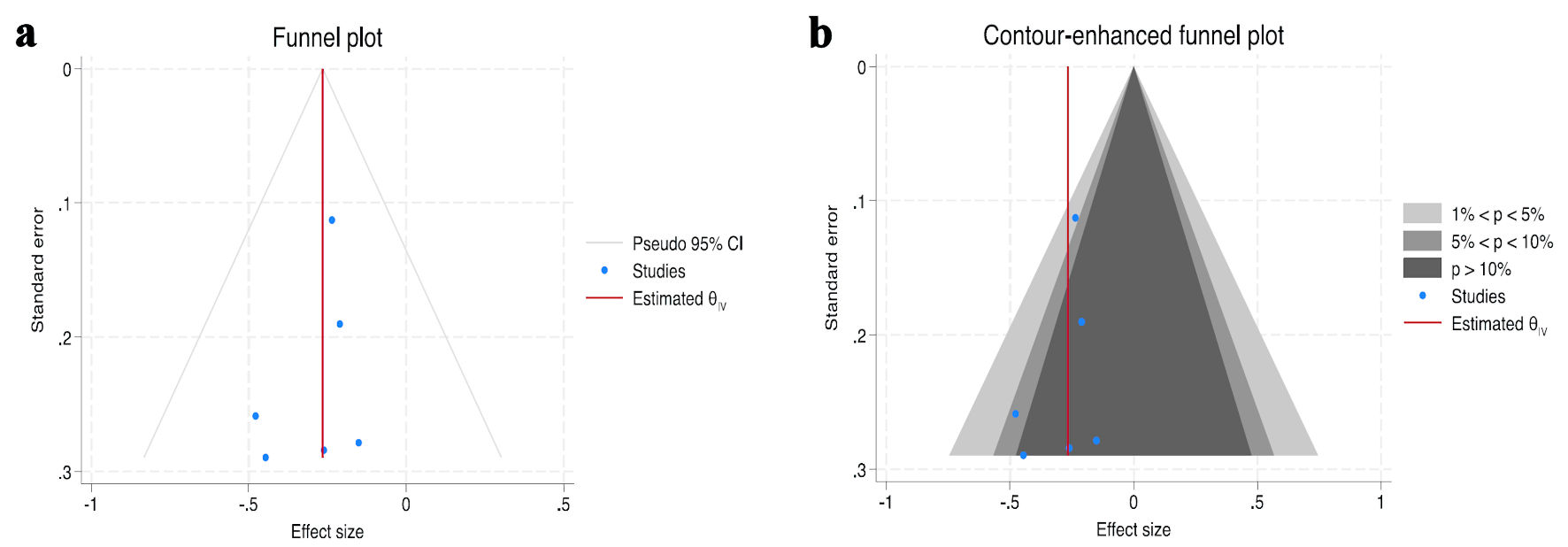 Click for large image | Figure 4. Funnel plot (a) and contour-enhanced funnel plot (b) of atrial fibrillation recurrence post-ablation studies included in meta-analysis. Egger’s test P value = 0.4175. CI: confidence interval; HFrEF: heart failure with reduced ejection fraction; HFmrEF: heart with mildly reduced ejection fraction; HFpEF: heart failure with preserved ejection fraction. |
| Discussion | ▴Top |
In this systematic review and meta-analysis including all three categories of HF, we found no difference in the rate of AF recurrence post-ablation among a cohort of majority ablation-naive patients with HFrEF, HFmrEF, or HFpEF with a recurrence rate of 40%, 35%, and 35%, respectively at a median follow-up of 24 months. These findings are in line with previous studies showing similar rate of AF recurrence post-ablation in HFrEF when compared to HFpEF [14-17]. To the best of our knowledge, this meta-analysis is the first to demonstrate similar efficacy of CA in those with HFmrEF compared to those with HFrEF and HFpEF.
Only two [18, 25] of the seven studies analyzed included outcomes of all-cause mortality or HF hospitalizations within all three categories of HF, limiting our ability to perform a robust comparative analysis for hard clinical endpoints in those with HFmrEF who undergo CA compared to HFrEF and HFpEF. Pooled analysis demonstrated no difference in composite all-cause mortality and HF hospitalizations in patients with HFmrEF and HFpEF, and the benefit in patients with HFrEF was driven largely by a reduction in HF hospitalizations. Caution should be used in interpreting this finding, as more data from future prospective studies investigating such clinical endpoints in all three HF categories are needed. While Fujimoto et al [23] and Eitel et al [22] evaluated all-cause mortality and HF hospitalizations among all three categories of HF, there was no control group on medical therapy, and therefore was not included in the pooled analysis. Fujimoto et al indicated an approximately three times higher incidence of composite all-cause mortality and HF hospitalizations, all-cause mortality alone, and HF hospitalizations alone among those with HFrEF, compared to those with HFmrEF and HFpEF [23]. These findings are discordant with that of Eitel et al [22], where there was no difference in composite all-cause mortality and HF hospitalization among the three HF subgroups. Data from Fujimoto et al [23] is aligned with at least one meta-analysis, which demonstrated higher mortality among patients with HFrEF who underwent AF ablation when compared to those with HFpEF who underwent AF ablation [16]. Rillig et al, in a subanalysis of the EAST-AFNET4 trial, demonstrated the clinical benefit of early rhythm control extended to those with HF across the three categories over a rate control strategy [25]. However, the early rhythm control strategy employed both ablation and AAD. The limited number of studies investigating clinical endpoints of AF ablation in comparison to medical therapy, namely antiarrhythmic therapy, across the three HF categories, and discordant results in mortality and HF hospitalizations among those with HFrEF, highlight the need for further clinical trial data.
Numerous studies have found that AF accentuates the risk of clinical outcomes such as mortality, HF hospitalization, and stroke among the combined HF population, and within individual HF subtypes, although there are conflicting data on increased mortality risk on patients with HFrEF and AF [3-6]. Aggregate data from several meta-analyses have shown that compared to conservative medical therapy, AF ablation is associated with significant reduction in AF recurrence, all-cause mortality, and HF hospitalization in patients with HFrEF [8-10]. In those with HFpEF, AF ablation is associated with a significant reduction in AF recurrence and may be associated with a reduction in all-cause mortality and HF hospitalizations when compared to conservative medical therapy [11-14]. The advent of HFmrEF as a distinct HF subtype was formally introduced in 2016 European Society of Cardiology Heart Failure Guidelines. Prior to this, there was a lack of consistency in definitions of HFrEF and HFpEF, with variable cutoffs for LVEF that would often overlap in different studies. A recent retrospective study by Lee et al evaluating the efficacy of AF ablation among patients with HFmrEF demonstrated a similar rate of AF recurrence (30.6%) at a mean of 22 months, as found in our analysis, and demonstrated a significant reduction in both all-cause mortality and HF hospitalizations when compared to medical therapy [26]. While this study was not included in our analysis because it lacked comparison to HFrEF and HFpEF subtypes, it highlights the potential benefit of AF ablation among those with HFmrEF. It is important that future clinical trials continue to investigate AF ablation in comparison to medical therapy among these subgroups to better delineate the potential impact on clinical outcomes across the HF spectrum.
Limitations
We observed some limitations. The included studies in this systematic review and meta-analysis were mainly observational cohort studies, registries, or post-hoc analyses and were subject to inherent biases and confounding variables from non-randomized sampling. There was heterogeneity within study protocols that could influence results and interpretation. For example, a blanking period was utilized in some of the included studies, typically ranging from 2 to 3 months, while several of the included studies [18, 22, 25] did not incorporate a blanking period. Additionally, there was variability with the use of AAD, as the majority of studies allowed continued use of AAD beyond the blanking period if applicable [18, 22, 23, 25]. While Chen et al [21] only allowed AAD through the duration of the blanking period, Yazaki et al [19] required AAD discontinuation prior to CA. The majority of included studies [19, 21-24] did not employ a comparative medical therapy group for either rate control or antiarrhythmic therapy, limiting our ability to compare the efficacy of CA to contemporary medical therapy in this patient cohort, and highlighting the need for further trial data.
Conclusions
CA has similar efficacy in the management of AF among patients with HFrEF, HFmrEF, and HFpEF. Currently, few studies comparing clinical endpoints such as all-cause mortality and HF hospitalizations among patients with HFrEF, HFmrEF, and HFpEF who undergo CA exist. These findings highlight a need for further prospective studies to evaluate the potential benefit of AF ablation compared to contemporary medical therapy, primarily rhythm control strategies with AAD, across the HF spectrum.
Acknowledgments
Please note that the work performed by Carl Hashem originally emanated from a previous affiliation with the Boston University Chobanian and Avedisian School of Medicine.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors have no conflict of interest to report.
Informed Consent
Not applicable.
Author Contributions
Carl Hashem: writing - original draft, review and editing. Matthew F. Yuyun: conceptualization, formal analysis, methodology, software, writing - review and editing. Jacob Joseph: writing - review and editing. Scott Kinlay: writing - review and editing. Adelqui O. Peralta: writing - review and editing. Peter S. Hoffmeister: writing - review and editing. Each author has made significant contributions to the development of this article. All authors have read and approved the final article.
Abbreviations
AAD: antiarrhythmic drugs; AF: atrial fibrillation; CA: catheter ablation; DM: diabetes mellitus; ECG: electrocardiogram; HF: heart failure; HFmrEF: HF with mildly reduced ejection fraction; HFpEF: HF with preserved ejection fraction; HFrEF: HF with reduced ejection fraction; HTN: hypertension; IHD: ischemic heart disease; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; PAF: paroxysmal atrial fibrillation; PerAF: persistent atrial fibrillation; RCT: randomized controlled trial; TIA: transient ischemic attack
| References | ▴Top |
- Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119(18):2516-2525.
doi pubmed - Mountantonakis SE, Grau-Sepulveda MV, Bhatt DL, Hernandez AF, Peterson ED, Fonarow GC. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of get with the guidelines-heart failure. Circ Heart Fail. 2012;5(2):191-201.
doi pubmed - Kroshian G, Joseph J, Kinlay S, Peralta AO, Hoffmeister PS, Singh JP, Yuyun MF. Atrial fibrillation and risk of adverse outcomes in heart failure with reduced, mildly reduced, and preserved ejection fraction: A systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2024;35(4):715-726.
doi pubmed - Odutayo A, Wong CX, Williams R, Hunn B, Emdin CA. Prognostic importance of atrial fibrillation timing and pattern in adults with congestive heart failure: a systematic review and meta-analysis. J Card Fail. 2017;23(1):56-62.
doi pubmed - Naka KK, Bazoukis G, Bechlioulis A, Korantzopoulos P, Michalis LK, Ntzani EE. Association between atrial fibrillation and patient-important outcomes in heart failure patients with implantable cardioverter-defibrillators: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes. 2019;5(2):96-104.
doi pubmed - Mundisugih J, Franke KB, Tully PJ, Munawar DA, Kumar S, Mahajan R. Prevalence and prognostic implication of atrial fibrillation in heart failure subtypes: systematic review and meta-analysis. Heart Lung Circ. 2023;32(6):666-677.
doi pubmed - Ravi V, Poudyal A, Lin L, Larsen T, Wasserlauf J, Trohman RG, Krishnan K, et al. Mortality benefit of catheter ablation versus medical therapy in atrial fibrillation: An RCT only meta-analysis. J Cardiovasc Electrophysiol. 2022;33(2):178-193.
doi pubmed - Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427.
doi pubmed - AlTurki A, Proietti R, Dawas A, Alturki H, Huynh T, Essebag V. Catheter ablation for atrial fibrillation in heart failure with reduced ejection fraction: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2019;19(1):18.
doi pubmed - Smer A, Salih M, Darrat YH, Saadi A, Guddeti R, Mahfood Haddad T, Kabach A, et al. Meta-analysis of randomized controlled trials on atrial fibrillation ablation in patients with heart failure with reduced ejection fraction. Clin Cardiol. 2018;41(11):1430-1438.
doi pubmed - Androulakis E, Sohrabi C, Briasoulis A, Bakogiannis C, Saberwal B, Siasos G, Tousoulis D, et al. Catheter ablation for atrial fibrillation in patients with heart failure with preserved ejection fraction: a systematic review and meta-analysis. J Clin Med. 2022;11(2):288.
doi pubmed - Rattka M, Kuhberger A, Pott A, Stephan T, Weinmann K, Baumhardt M, Aktolga D, et al. Catheter ablation for atrial fibrillation in HFpEF patients-A propensity-score-matched analysis. J Cardiovasc Electrophysiol. 2021;32(9):2357-2367.
doi pubmed - Jiang H, Su H Y, Tan V H, Yeo C. Catheter ablation vs medical therapy for treatment of atrial fibrillation patients with heart failure with preserved ejection fraction: a systematic review and meta-analysis. European Heart Journal. 2023;44(Supplement_1):ehac779.020.
- Arora S, Jaswaney R, Jani C, Zuzek Z, Thakkar S, Patel HP, Patel M, et al. Catheter ablation for atrial fibrillation in patients with concurrent heart failure. Am J Cardiol. 2020;137:45-54.
doi pubmed - Siddiqui MU, Junarta J, Riley JM, Ahmed A, Pasha AK, Limbrick K, Alvarez RJ, et al. Catheter ablation in patients with atrial fibrillation and heart failure with preserved ejection fraction: A systematic review and meta-analysis. J Arrhythm. 2022;38(6):981-990.
doi pubmed - Aldaas OM, Lupercio F, Darden D, Mylavarapu PS, Malladi CL, Han FT, Hoffmayer KS, et al. Meta-analysis of the usefulness of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2021;142:66-73.
doi pubmed - Lopatin Y. Heart failure with mid-range ejection fraction and how to treat it. Card Fail Rev. 2018;4(1):9-13.
doi pubmed - von Olshausen G, Benson L, Dahlstrom U, Lund LH, Savarese G, Braunschweig F. Catheter ablation for patients with atrial fibrillation and heart failure: insights from the Swedish Heart Failure Registry. Eur J Heart Fail. 2022;24(9):1636-1646.
doi pubmed - Yazaki K, Ejima K, Kataoka S, Higuchi S, Kanai M, Yagishita D, Shoda M, et al. Prognostic significance of post-procedural left ventricular ejection fraction following atrial fibrillation ablation in patients with systolic dysfunction. Circ Rep. 2020;2(12):707-714.
doi pubmed - Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316.
doi pubmed - Chen C, Cheng K, Gao X, Zou T, Pang Y, Ling Y, Xu Y, et al. Cryoballoon ablation for atrial fibrillation in patients with heart failure with mildly reduced and preserved ejection fraction. ESC Heart Fail. 2023;10(1):518-531.
doi pubmed - Eitel C, Ince H, Brachmann J, Kuck KH, Willems S, Gerds-Li JH, Tebbenjohanns J, et al. Atrial fibrillation ablation strategies and outcome in patients with heart failure: insights from the German ablation registry. Clin Res Cardiol. 2019;108(7):815-823.
doi pubmed - Fujimoto H, Doi N, Okayama S, Naito M, Kobori A, Kaitani K, Inoue K, et al. Long-term prognosis of patients undergoing radiofrequency catheter ablation for atrial fibrillation: comparison between heart failure subtypes based on left ventricular ejection fraction. Europace. 2022;24(4):576-586.
doi pubmed - Mekhael M, Shan B, Noujaim C, Chouman N, Assaf A, Younes H, El Hajjar AH, et al. Catheter ablation improved ejection fraction in persistent AF patients: a DECAAF-II sub analysis. Europace. 2023;25(3):889-895.
doi pubmed - Rillig A, Magnussen C, Ozga AK, Suling A, Brandes A, Breithardt G, Camm AJ, et al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation. 2021;144(11):845-858.
doi pubmed - Lee DY, Chang TY, Chang SL, Lin YJ, Lo LW, Hu YF, Chung FP, et al. Clinical outcomes and structural remodelling after ablation of atrial fibrillation in heart failure with mildly reduced or mid-range ejection fraction. ESC Heart Fail. 2023;10(1):177-188.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.