| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Review
Volume 15, Number 6, December 2024, pages 415-424
A Promising Pathway Toward Mitigation and Eradication of Coronary Artery Disease
Ronald P. Karlsberga, b, c, e, Geoffrey W. Chob, c, Jairo Aldana-Bitarc, d
aCedars Sinai Heart Institute, Los Angeles, CA, USA
bUniversity of California Los Angeles David Geffen School of Medicine, Los Angeles, CA, USA
cCardiovascular Research Foundation of Southern California, Beverly Hills, CA, USA
dThe Lundquist Institute at Harbor-UCLA, Torrance, CA, USA
eCorresponding Author: Ronald P. Karlsberg, Cardiovascular Research Foundation of Southern California, Beverly Hills, CA, USA
Manuscript submitted August 24, 2024, accepted November 8, 2024, published online December 3, 2024
Short title: Advancing Coronary Artery Disease Eradication
doi: https://doi.org/10.14740/cr1721
- Abstract
- Introduction
- CCTA
- Polygenic Risk Scores (PRSs)
- Clonal Hematopoiesis
- Telomeres
- AI in Cardiac Imaging
- Ribonucleic Acid (RNA) Therapeutics
- Discussion
- References
| Abstract | ▴Top |
Cardiovascular disease remains the leading cause of death in the United States and globally. Significant advances have been made throughout the history of cardiology and the treatment of this disease; however, these efforts have not halted the alarming statistics. Emerging approaches, such as artificial intelligence applied to cardiac imaging, genetic testing, and genetic silencing, may offer essential additional steps in treating the disease. Moreover, new pathways of the disease are being identified, which differ from traditional risk factors and offer a fresh, innovative approach. This paper focuses on a novel strategy that includes identifying and treating multiple pathways of the disease using both new and traditional interventions. These interventions include plaque-directed therapy rather than surrogate therapy, with the potential to mitigate consequences and possibly eradicate the disease through personalized, multi-approach treatments similar to those used in cancer treatment.
Keywords: Coronary artery disease; Artificial intelligence; RNA; Clonal hematopoiesis; Plaque; Genome wide association studies; Polygenic risk score; Cardiac computed tomography
| Introduction | ▴Top |
We aim to describe a pathway that may lead to better management, mitigation, and eradication of coronary artery disease (CAD) and is consequences. Like many other advances, this possibility comes from the integration of advances and technologies that present radical changes and new opportunities.
It has been over 200 years since the description of the clinical presentation of diseased coronary arteries, angina pectoris [1], by John Warren. It took decades and evolving advances to understand its relationship to coronary artery narrowing, how the balance between oxygen supply and demand results in the clinical presentation of chest pain, and how an acute obstruction leads to myocardial infarction. Invasive coronary artery angiography enabled real-time visualization of diseased coronary arteries, but it took decades before we focused on bypassing the disease and addressing the actual cause of angina: narrowing of the coronary arteries.
The tools of angioplasty, followed by stenting and eventually drug-eluting stents, were developed over the course of decades. Finally, the actual cause of disease could be visualized using intravascular ultrasound (IVUS) and optical coherence, which finally were able to characterize the cause of disease that corresponded to known histology. Simultaneously, computed tomography (CT) has evolved over the past five decades. When we initially used CT with primitive devices, we captured coronary arteries for the first time on Polaroid paper with minimal resolution [2]. With the development of computed tomographic coronary angiography (CCTA), also known as coronary computed tomographic angiography (CTA) and its use, even without symptoms, we began to understand and finally monitor the disease from the development of non-calcified plaque [3] to calcification and narrowing [4]. Finally, tools based on augmented intelligence [5] and correlation to intracoronary hemodynamics [6-8] has led to predicting the need for revascularization [9, 10]. Coronary CTA has augmented and even replaced invasive angiography, as well as the ability to use medications to directly treat and monitor the disease, rather than rely on surrogate measures and predicting downstream major adverse cardiac events (MACEs) [11, 12].
With the simultaneous development of tools to understand the human genome and recent advances in gene manipulation and disease expression, we now have the opportunity to address the long-standing family history component - once considered an unmodifiable risk factor - through genetic intervention [13]. Taking together, these tools reinforce our existing understanding that we can mitigate disease by addressing modifiable risk factors, using medications that affect the pathways of the disease, and now, with genetic manipulation, we can create a new and possible final platform of tools and knowledge to eliminate the disease. One approach is to focus on the early identification of diseased arteries when the condition first appears, rather than treating all at-risk individuals with established methods. Many individuals with risk factors, elevated cholesterol, and family histories never develop the disease because the multiple pathways for atherosclerosis may not be present due to genetic variations. Individuals with no calcified or non-calcified plaques tend to have excellent outcomes regardless of treatment. However, if individuals are treated based solely on risk factors, they are exposed to potential side effects without any improvement in their outcomes. Additionally, new methods to silence the genes related to the disease offer a promising strategy. The focus is shifting from surrogate and indirect measures of the disease to directly monitoring the beginning and evolution of plaque directly by the use of coronary CTA.
Visualization of plaque in asymptomatic patients and the use of machine learning and artificial intelligence (AI) to directly measure and characterize the consequences of plaque with serial measurements, present the opportunity for individual and personalized treatments. With this personalized approach, the multiple pathways of CAD and subsequent treatments may be evaluated by determining their effects on the evolution of plaque for each person. This will enable the use of personalized treatments to target modifiable risk factors, incorporating emerging gene-silencing techniques and outcome metrics like MACEs [14]. Evolving research is ongoing to integrate these developments to establish a fresh evidence base for the treatment of CAD [15].
Guidelines for personalized precision medicine tailored to each patient’s unique health needs and risks, whether high or low, are still lacking. However, recent reviews have begun to focus on this approach [11]. For example, in the Get With The Guidelines study, among 136,905 patients admitted for CAD, half had admission low-density lipoprotein (LDL) levels of < 100 mg/dL, and 17.6% presented with LDL of < 70 mg/dL. Despite controlled LDL levels, cardiac events still occurred in these patients [16]. Furthermore, the FACTOR-64 trial, which evaluated coronary CTA in patients with diabetes and CAD, did not show a reduction in major cardiac events [17]. Managing risk factors alone is insufficient to prevent adverse cardiac outcomes. We will address the elements of a more comprehensive treatment approach in this review.
| CCTA | ▴Top |
Coronary CTA performs comprehensive whole-heart analysis, and in particular is able to provide in-depth examination of plaque in the coronary arteries [18]. A recent consensus that graded various imaging modalities for atherosclerosis determined that coronary CTA is the most appropriate technique for atherosclerotic plaque evaluation [19]. Both standard and adaptive quantification/qualification of plaque and its subtypes can be performed, and recently it is even performed in an automated fashion with the use of various AI, machine learning, and deep learning programs [20, 21]. Robust data are emerging that plaque subtypes and atherosclerotic plaque characteristics may further risk-stratify patients, and proposals which specifically evaluate in-depth plaque are now being considered in several large clinical trials, replacing the traditional focus on MACE in previous studies [11, 22]. The plaque-based approach implements whole heart atherosclerosis phenotyping via CTA, which can allow for precision medicine by individualizing and staging each specific patient’s disease. For instance, AI-driven CTA can evaluate and grade each patient’s plaque subtypes, identify metrics such as atheroma volume, differentiate percentage of soft versus calcified plaque, and track these changes serially to determine the velocity of disease and/or the efficacy of treatments. In addition, coronary CTA is able to collect this information with fewer risks than invasive coronary angiography [23]. These AI-based CTA grading systems have demonstrated prognostic value and the ability to reclassify risk in a recent 10-year follow-up study, with an event rate approaching 0% in individuals without atherosclerosis [24].
It is also vital to consider the practical applications regarding mass utilization of CT screening on a population level. The ongoing TRANSFORM and TRANSCEND trials are currently evaluating several aspects of this, assessing serial CCTA monitoring of patients without any established CAD at baseline, followed by individualized plaque reduction therapy and assessment of long-term safety and costs related between treatment groups versus standard of care. In addition, several countries already engage in mass screening with CT/magnetic resonance imaging (MRI) on a population base. For instance, Japan has the highest number of both MRI and CT scanners per capita of any Organization for Economic Cooperation and Development (OECD) country. All citizens are recommended to undergo whole body MRI and/or CT screening starting around age 40 - 50 as part of their general health screening program. This is in part due to not only their nationalized healthcare system but also several clinical trials of proven cost-effectiveness showing the benefit of early disease identification and treatment by population imaging.
As written by Burch et al [25], studies have shown that CCTA is a cost-effective strategy for both initial and serial CAD evaluation. Even in low-to-intermediate risk patients, analysis suggests the potential to reduce economic costs with better health outcomes compared to current standard of care testing. Of course, this does not obviate the need for continued prospective trials in the future to continue to evaluate the practicality, safety, radiation risk and economic burden of serial imaging; but so far, it is promising to see multiple trials suggesting that the use of CCTA can be a cost-effective and outcome improving strategy for CAD prevention and management [25].
It is established that CAD is driven by plaque; however, the precise pathways of the various subtypes and the factors that contribute to the natural progression of the disease remain to be elucidated. The clarification of the multiple pathways of plaque production, and the ability to serially measure the derived metrics offer a pathway to the mitigation and eradication of the disease. Beyond LDL manipulation and evaluating surrogate measures, a focus on multiple pathways of the disease, in combination with plaque-directed therapies, offers hope for improving our current approaches toward eradicating the disease. These potential pathways are shown in Figure 1.
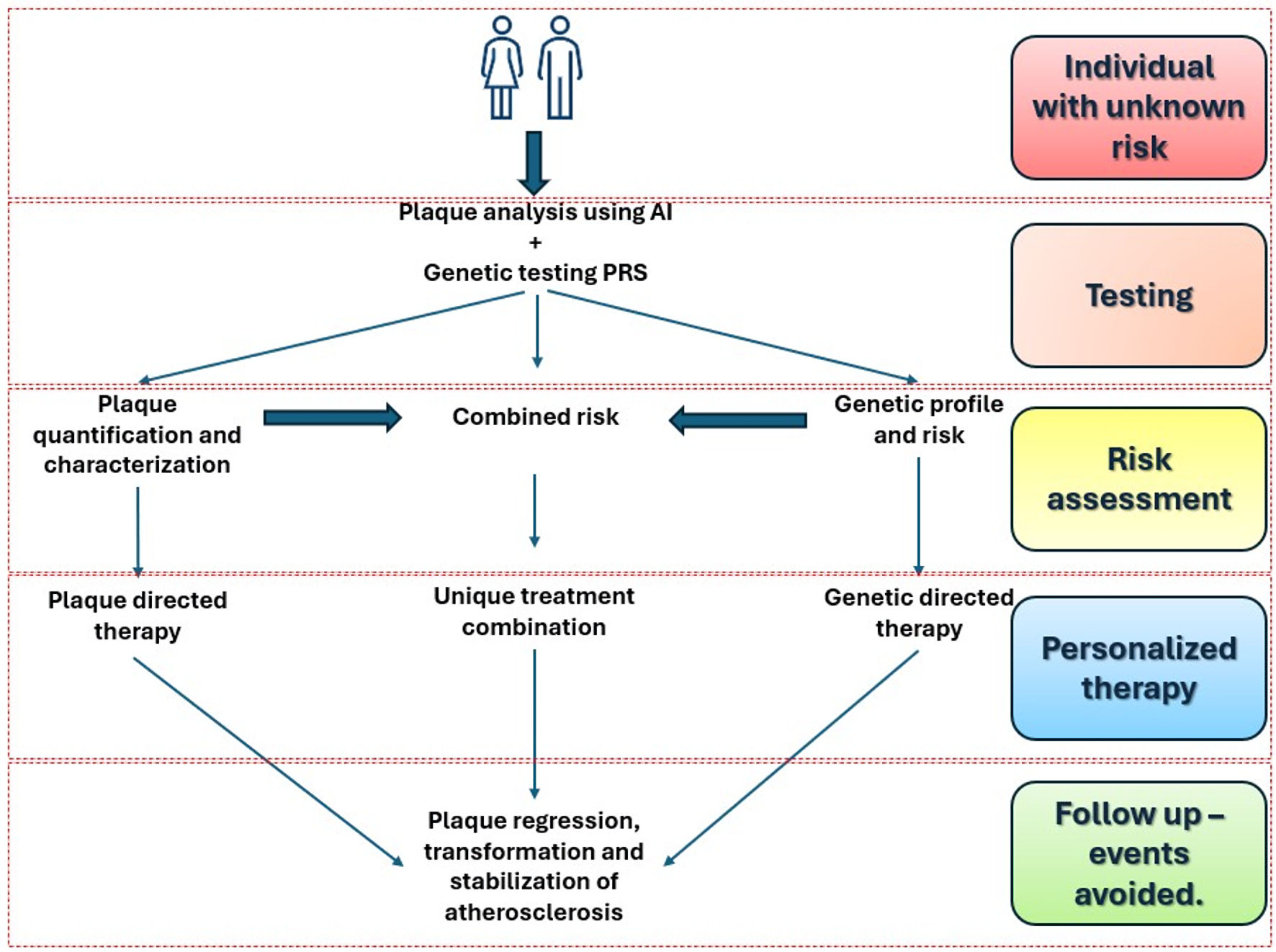 Click for large image | Figure 1. Summary of the pathway for the eradication of CAD. This general overview begins with identifying individuals with unknown risk, followed by testing coronary CTA and AI-based plaque analysis, combined with genetic profiling using a polygenic risk score (PRS) to assess risk. The next step is personalized therapy, concluding with ongoing follow-up to prevent future events. AI: artificial intelligence; CAD: coronary artery disease; CTA: computed tomographic angiography. |
| Polygenic Risk Scores (PRSs) | ▴Top |
Multiple lines of evidence suggest that the development of CAD can be partially predicted through genetic testing via genome-wide association studies (GWAS), with these genetic factors accounting for up to 50% of the disease [26]. The silencing of these influences, in combination with our current lifestyle approaches and pharmacological treatments, together with plaque-derived endpoints, represents an evolving pathway. In particular, the single-nucleotide polymorphisms identified by GWAS that cause and predispose individuals to CAD, refine our knowledge of genetic sequences and amplify the personalized approach to treatments [27]. For example, the personalized risk scores derived from GWAS have demonstrated utility in multiple fields, including preeclampsia/gestational hypertension [28], thoracic aortic aneurysms [29], and more recently also in CAD. Studies as part of the CARDIoGRAMplusCD4 Consortium, Biobank Japan, and EPIC-CVD, in particular, have provided evidence to suggest that several hundred loci may be mechanistically instrumental in the development of CAD. Furthermore, treatments that target these genes (e.g., with methods such as CRISPR-Cas9) may have the potential to prevent and treat CAD on an individual patient level [30-32]. Further investigation of PRS will continue to refine the genetic factors that drive CAD. The elucidation of the relevant genetic loci offers a laser and personalized approach that will define the multiple required treatment tailored and personalized to address the individuals’ unique multifactorial causes of CAD. Whether the presence of genetic risk is the first notice to start intensive therapy, or the phenotypic expression of disease with non-calcified plaque or calcified plaque is the starting point for aggressive therapy, remains unclear. However, a genetic test that has a high likelihood of predicting phenotypic disease may be simpler and have more widespread application than plaque imaging with coronary CTA.
| Clonal Hematopoiesis | ▴Top |
While it is well established that the incidence of cardiac events increases with age, the precise mechanisms by which aging contributes to CAD are still not fully understood. Clonal hematopoiesis is a process of cell replication and expansion, which if dysregulated (via introduction of a mutation), will lead to subsequent production of pathologic cells that differ from a patient’s normal occurring blood line. Often as a topic of investigation in the oncology field, it is also known that it can also result by dysfunction of hematopoietic stem cells with aging. This occurs within the “inflamm-ageing” process of persistent low-grade inflammation over time, with elevated levels of inflammatory cytokines such as interleukin (IL)-6, IL-1β and tumor necrosis factor, negatively affecting the bone marrow microenvironment and disrupting cell linage and differentiation [33]. The presence of mutations leading to clonal hematopoiesis rises proportionally with age, with a prevalence of 18.4% in the oldest population, with the most common mutated genes identified so far being DNMT3A, TET2, ASXL1, JAK2, and TP53 [34]. These types of mutations have been called “clonal hematopoiesis of indeterminate potential” (CHIP) and are thought to be a significant risk factor to drive the increase degree of CAD with aging. As CHIP occurs, it activates the inflammasome, resulting in further inflammation and downstream progression of atherosclerosis [35]. Further understanding of the mechanisms of CHIP and how to prevent pathologic clonal hematopoiesis should also contribute to precision therapeutics for CAD prevention measured with plaque metrics.
| Telomeres | ▴Top |
Telomeres are short repetitive deoxyribonucleic acid (DNA) strands at the ends of chromosomes that help protect them from degradation. With each replication over time, they become shorter; and after reaching a critical length, they signal the DNA strand for degradation. When pathologically shortened or maintained beyond normal time limits, telomeres have been associated with a variety of conditions such as CAD and cancer. Progressive or accelerated rates of shortening have been implicated in biological aging [36] and may play a significant role in cardiovascular pathology. In a study of patients with heart failure, telomere length was measured in circulating leukocytes and was found to be significantly shorter than in controls [37]. Also, individuals with CAD have been shown to have shorter telomer length compared to healthy patients [38]. The ability to regulate telomerase activity, to potentially limit pathological changes by telomerase reverse transcriptase interventions, is being investigated. However, as telomere length is also significantly related to conditions including malignancy and genetic disorders as well, there exists a legitimate concern of cancer induction, which is a limitation in the development of this therapy [39]. The Telomerase Activator to Reverse Immunosenescence in Acute Coronary Syndrome (TACTIC) trial, a double-blind phase II pilot randomized controlled trial, investigating telomerase intervention in acute coronary syndrome, is awaiting publication [40]. We anticipate that subsequent studies evaluating telomeres in other areas of cardiac disease, such as heart failure, will provide further insight into the utility of this potential therapeutic modality. It will be feasible to use coronary CTA as a tool to study telomere interventions.
| AI in Cardiac Imaging | ▴Top |
Coronary CTA is a noninvasive imaging modality that allows in-depth analysis of coronary artery stenosis, plaque analysis, volume composition and quantification, and fractional flow reserve [18, 41]. Coronary CTA has been used across multiple pharmacological and non-pharmacological interventional trials to evaluate plaque regression and stabilization [11]. It is well established that plaque composition, and particularly plaques with high-risk features, is an important predictor of future cardiovascular events. Low attenuation plaque, defined as ≤ 30 Hounsfield units (HU) plaque, is lipid-rich, has a necrotic core, and is hypoechoic on IVUS [42.] In a post-hoc analysis of the SCOT-HEART (Scottish Computed Tomography of the Heart) trial, low attenuation plaque was found to be the strongest predictor of fatal and nonfatal myocardial infarction among cardiovascular risk scores, including coronary artery calcium score or obstructive coronary artery stenosis [43]. Large registries such as the CONFIRM have demonstrated the prognostic utility of coronary CT, the stenosis grading, and plaque visualization [44].
Coronary CT technology has been continually improving and evolving, becoming a central tool for noninvasively investigating coronary plaque. With advances in CT innovation, spatial resolution has improved from 0.400 - 0.500 mm in conventional CT to 0.150 - 0.200 mm range in ultra-high-resolution CT with photon counting [45]. Thus, with enhanced spatial resolution, we can anticipate even greater accuracy when combining advances in AI and software to implement plaque-directed therapy [46].
Furthermore, the integration of flow measurements derived from CT imaging has proven to be of vital clinical utility [12]. The PRECISE trial, a pragmatic, randomized clinical trial, evaluated a CT-driven precision strategy - risk-guided, patient-specific testing deferral for minimal-risk patients combined with coronary CTA and selective fractional flow reserve computed tomography (FFRCT) - against usual care. The trial demonstrated that the use of coronary CTA decreased the number of unnecessary catheterizations and improved the accuracy of identifying the need for invasive coronary angiograms for obstructive CAD in the precision strategy arm, compared to usual care [47].
In the rapidly emerging field of AI-driven coronary CTA, studies have shown that, in addition to enabling high throughput and rapid analysis of big data, it provides more accurate and consistent serial evaluations of CAD than human readers can achieve. The CLARIFY (CTEvaluation by Artificial Intelligence for Atherosclerosis, Stenosis and Vascular Morphology) studies has demonstrated that AI applied to coronary CTA is equal or superior to human experts, near-infrared spectroscopy (NIRS), IVUS, and quantitative coronary angiography (QCA) [5, 48, 49]. Ongoing registries such as CONFIRM-2 [50], among others are ongoing to help clarify the significant potential of AI in coronary CTA to contribute to improved clinical decision making and patient outcomes. The CERTAIN trial demonstrated the effectiveness of the improved diagnostic accuracy while reducing unnecessary downstream utilization and invasive testing, by using machine learning and AI [51]. The large randomized controlled trial TRANSFORM (ClinicalTrials.gov Identifier: NCT06112418), will evaluate the use of plaque analysis and quantification as primary prevention strategy with the use of multiple plaque modifying drugs [15]. Results are expected to be released in 2028 or before.
Pericoronary fat exerts a paracrine function around the coronary arteries, secreting inflammatory cytokines that closely interact with the vascular wall. The CT measurement tool is called the fat attenuation index (FAI) (-190 to -30 HU) [52], and a recent metanalysis showed that increased FAI was associated with increased MACE risk, making this a promising prognostication modality and also a therapeutic end point [53] even from pre-contrast scans [54]. Recently published, FAI demonstrated in the ORFAN study to be prognostic in a large-scale population, suggesting also that this could be an alternative and addition to standard risk evaluation [55].
Some have raised concern about radiation exposure when considering plaque-directed therapy. As shown by Nassenstein’s group, CCTA AI programs, such as those utilizing deep neural networks, can fully automate and enhance CT scan ranges, thereby lessening the effort needed to be performed by the staff and more importantly, reducing radiation exposure and improving patient safety [56]. In fact, larger trials such as the “PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice (PROTECTION VI)” showed that there has been dramatic improvement with radiation dose reduction in CCTA on an international scale, such that utilization of exposure dose lowering strategies which makes CCTA feasible even in daily practice [57].
Similarly, a large randomized clinical trial, the “Lower Radiation Dosing in Cardiac CT Angiography: The CONVERGE Registry” demonstrated that high resolution scanners, (e.g., 256 - row scanners or higher), afforded significantly less radiation and contrast dosing than 64-row scanners across multiple variables (e.g., body habitus, body mass index (BMI), etc.); overall providing better diagnostic accuracy with improved patient exposure safety [58]. More recently, the emerging technology revolution of photon-counting CT (PCCT) promises even higher resolution images with less radiation, needing not only less scanning time than conventional CTs, but vastly improved image resolution and quality, with reduced radiation dosing. Preliminary studies suggest that PCCT provides improved downstream diagnostic ability, reduced radiation, and comfort for patients, though further studies to see its effect on health outcomes and safety remain to be established.
| Ribonucleic Acid (RNA) Therapeutics | ▴Top |
RNA or DNA therapies are composed of exogenous sequences of nucleic acids. These molecules can regulate a wide variety of biological functions and are specific in their actions due to their uniquely programmed genetic sequence targets. Various modalities exist for treatments with RNAs, such as antisense oligonucleotide (ASO), RNA interference (RNAi) for gene silencing, small interfering RNAs (siRNAs), microRNAs (miRNAs), and RNA aptamers. Messenger RNA (mRNA) therapeutics are also used heavily in cardiovascular research and medicine, including agents such as mipomersen (ASO), pelacarsen (ASO), inclisiran (siRNA), olpasiran (siRNA) [59], among others.
For example, efforts have been made for reversing myocardial ischemia with vascular endothelial growth factor RNA-based therapies in heart failure associated with Chagas disease, among others [59]. RNA-based gene therapies via viral vectors have also shown to be helpful for cardiomyocyte survival and proliferation in myocardial injury models and are translating to clinical trials that have shown early safety and efficacy. This therapy modality has also opened a new door for cardiovascular “vaccines” aimed at prevention and treatment of heart disease, with further research trials currently ongoing. Finally, miRNA-based therapies are emerging as promising treatment avenues for atherosclerosis [60], especially following the recognition of this field with the 2024 Nobel Prize. A summary of the mechanisms and approach is shown in Figure 2.
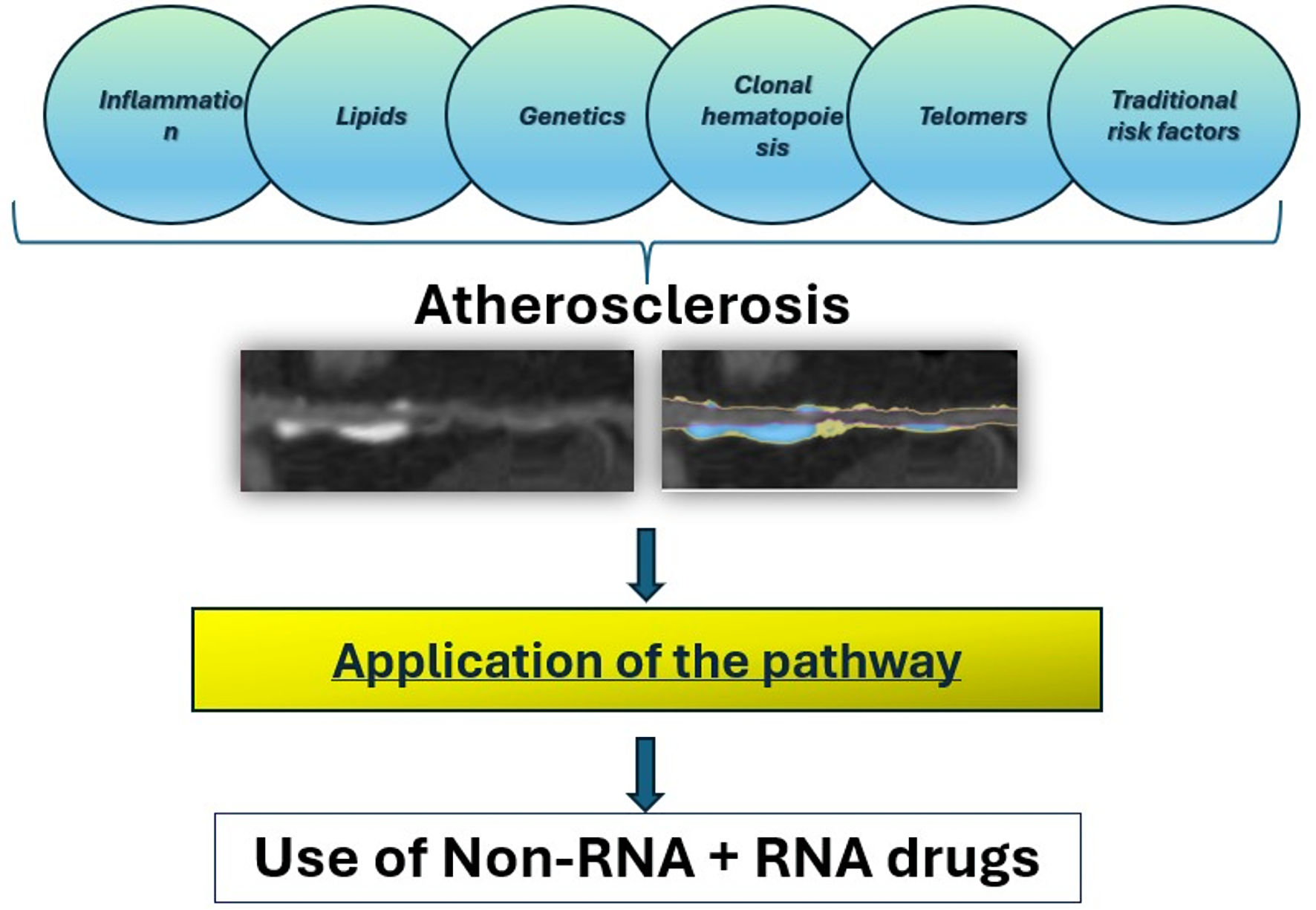 Click for large image | Figure 2. Summary of multiple contributors to atherosclerosis and CAD, also a simple but comprehensive flowchart is proposed with the use of personalized combination of medications using non-RNA and RNA drugs. CAD: coronary artery disease; RNA: ribonucleic acid. |
| Discussion | ▴Top |
Despite it being over two centuries since the description of angina pectoris, CAD remains a foremost global cause of death. Current approaches primarily focus on population-wide risk factors, which have reduced mortality. However, studies clearly demonstrate the need for more personalized medicine to tailor treatments to individuals, as population data are often inadequate to address each patient’s unique causes of the disease. Shifting towards the personalized approach that considers the genetic, environmental, and lifestyle factors unique to each patient will include methods which identify genetic markers that may predispose individuals to more aggressive forms of plaque formation, and tailoring interventions accordingly.
Methodological advancements outlined in this article are reshaping our understanding and treatment of CAD. Coronary CTA and the direct visualization of plaque formation, composition, and progression in the coronary arteries allows for more precise assessment of the risk and extent of CAD. Inflammation plays a critical role in the development and progression of atherosclerotic plaque. Investigating treatments that promote the stabilization or regression of plaque and targeting it from multiple facets via lifestyle changes, pharmaceuticals, and vaccines like treatments offer the potential to limit development of CAD. Implementing these strategies requires a multidisciplinary approach, integrating cardiology, genetics, imaging, and personalized medicine to shift the focus from merely controlling risk factors and cholesterol levels and other surrogate measures to actively prevent the driving causes of CAD.
The approach of plaque-directed therapy with the wide range of personalized treatments, including existing lifestyle medications and genetic silence, is audacious but feasible. It is important to recognize the limitations in translating these advances into widespread clinical practice. It may be unrealistic to monitor disease progression, as envisioned globally. Rather, once the pathways are defined for wide scale application, we will need to develop a more cost-effective and pragmatic approach.
As recently published in the European Heart Journal of Quality Care Clinical Outcomes, these studies demonstrated that compared with standard management, utilization of AI-augmented CCTA risk assessment optimizes cost effectiveness and improves cardiac treatment outcomes [61]. In the UK, CCTA is already a first-line test for CAD evaluation. However, it is well known that cardiac events can occur even without obstructive CAD. Ongoing studies, such as those currently done at the University of Oxford, are assessing the long-term cost-effectiveness, safety, and health outcomes of routine use of AI-augmented CCTA, which will hopefully further establish the ongoing benefit of improving patient outcomes though serial imaging [61]. The American Heart Association has also published a recent statement in 2024, titled “Value Creation Through Artificial Intelligence and Cardiovascular Imaging”, which emphasizes the importance of making a framework of CCTA AI utilization, and outlines the various ways it has already shown potential for improving diagnosis, management and reducing cardiovascular events via a safe, cost-efficient real-world setting [62]. AI approaches have moved to predicting standard of care guided revascularization [63].
Thus, we also await the development of tools that are less expensive, involve less radiation, and are more widely available. Highly specific and sensitive genetic testing, combined with AI-based analysis that includes modifiable and traditional risk factors and accurately prognosticates disease development, including the 50% influenced by genetics, remains a missing link that could reduce dependence on serial imaging to achieve personalized treatment plans.
For now, the integration of these technologies with plaque-directed endpoints promises to revolutionize CAD management, providing a comprehensive pathway to combat heart disease effectively using a multifaceted approach (Fig. 1). The culmination of all these emerging therapeutics including vaccine like therapies, will allow physicians to remain a step ahead of CAD, allowing not only improvements in treatment, but more importantly, leading to prevention of heart disease. Instead of managing surrogate measures, there is continued optimism we will be able to identify patients before onset of disease at the preventive stage, and treating its earliest signs. Finally, by addressing the multiple pathological pathways, identifying and treating genetic pathways, tailoring treatments to individual needs, and tracking these changes over time with new tools such as AI [4, 64], we have delineated a strategy to mitigate the consequences of CAD and possibly eradicate the disease itself.
Acknowledgments
None to declare.
Financial Disclosure
No funding was provided for this manuscript.
Conflict of Interest
The authors declare no competing interest.
Author Contributions
All authors contributed equally. Ronald P. Karlsberg is the guarantor of the paper. Ronald P. Karlsberg conceived and designed the study, conducted the literature search, developed the concepts and pathways described, wrote the manuscript, managed all edits, rewrote the final draft, answered all the editors’ questions, and handled all correspondence. Jairo Aldana-Bita wrote the first draft after receiving the theories, provided the illustrations, supplied the initial set of references, and approved the final draft. Geoffrey W. Cho edited one draft; made additions; assisted in answering questions of reviewers and approved the final draft.
Data Availability
No datasets were generated or analyzed during the current study. The authors declare that data supporting the findings of this study are available within the article. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author Ronald P. Karlsberg.
Abbreviations
AI: artificial intelligence; ASO: antisense oligonucleotide; CAD: coronary artery disease; CCTA: coronary computed tomographic angiography; CHIP: clonal hematopoiesis of indeterminate potential; CLARIFY: CTEvaluation by Artificial Intelligence for Atherosclerosis, Stenosis and Vascular Morphology; CT: computed tomography; CTA: computed tomographic angiography; DNA: deoxyribonucleic acid; FAI: fat attenuation index; GWAS: genome-wide association studies; IVUS: intravascular ultrasound; MACEs: major adverse cardiac events; miRNA: microRNA; MRI: magnetic resonance imaging; mRNA: messenger RNA; NIRS: near-infrared spectroscopy; OECD: Organization for Economic Cooperation and Development; PCCT: photon-counting computed tomography; PRS: polygenic risk score; QCA: quantitative coronary angiography; RNA: ribonucleic acid; RNAi: RNA interference; SCOT-HEART: Scottish Computed Tomography of the Heart; siRNA: small interfering RNAs; TACTIC: Telomerase Activator to Reverse Immunosenescence in Acute Coronary Syndrome
| References | ▴Top |
- Warren J. Remarks on angina pectoris. The New England Journal of Medicine, Surgery and Collateral Branches of Science. 1812;1(1):1-11.
- Karlsberg RP, Sagel SS, Ferguson TB. Myocardial infarction due to tumor embolization following pulmonary resection. Chest. 1978;74(5):582-584.
doi pubmed - Madaj PM, Budoff MJ, Li D, Tayek JA, Karlsberg RP, Karpman HL. Identification of noncalcified plaque in young persons with diabetes: an opportunity for early primary prevention of coronary artery disease identified with low-dose coronary computed tomographic angiography. Acad Radiol. 2012;19(7):889-893.
doi pubmed - Cho GW, Anderson L, Quesada CG, Jennings RS, Min JK, Earls JP, Karlsberg RP. Serial analysis of coronary artery disease progression by artificial intelligence assisted coronary computed tomography angiography: early clinical experience. BMC Cardiovasc Disord. 2022;22(1):506.
doi pubmed - Choi AD, Marques H, Kumar V, Griffin WF, Rahban H, Karlsberg RP, Zeman RK, et al. CT evaluation by artificial intelligence for atherosclerosis, stenosis and vascular morphology (CLARIFY): a multi-center, international study. J Cardiovasc Comput Tomogr. 2021;15(6):470-476.
doi pubmed - Nurmohamed NS, Danad I, Jukema RA, de Winter RW, de Groot RJ, Driessen RS, Bom MJ, et al. Development and validation of a quantitative coronary CT angiography model for diagnosis of vessel-specific coronary ischemia. JACC Cardiovasc Imaging. 2024;17(8):894-906.
doi pubmed - Helmy SA, Al-Attiyah RJ. The immunomodulatory effects of prolonged intravenous infusion of propofol versus midazolam in critically ill surgical patients. Anaesthesia. 2001;56(1):4-8.
doi pubmed - Karlsberg RP, Nurmohamed NS, Quesada CG, Samuels BA, Dohad S, Anderson LR, Crabtree T, et al. Performance of an artificial intelligence-guided quantitative coronary computed tomography algorithm for predicting myocardial ischemia in real-world practice. Int J Cardiol Heart Vasc. 2024;53:101433.
doi pubmed - Karlsberg RP, Budoff MJ, Thomson LE, Friedman JD, Berman DS. Reduction in downstream test utilization following introduction of coronary computed tomography in a cardiology practice. Int J Cardiovasc Imaging. 2010;26(3):359-366.
doi pubmed - Karlsberg RP, Budoff MJ, Berman DS, Thomson LE, Friedman JD. 64-slice multidetector computed tomography (MDCT) reduces other diagnostic studies for coronary artery disease. Am J Med. 2009;122(11):e13.
doi pubmed - Aldana-Bitar J, Bhatt DL, Budoff MJ. Regression and stabilization of atherogenic plaques. Trends Cardiovasc Med. 2024;34(5):340-346.
doi pubmed - Packard RR, Li D, Budoff MJ, Karlsberg RP. Fractional flow reserve by computerized tomography and subsequent coronary revascularization. Eur Heart J Cardiovasc Imaging. 2017;18(2):145-152.
doi pubmed - German DM, Mitalipov S, Mishra A, Kaul S. Therapeutic genome editing in cardiovascular diseases. JACC Basic Transl Sci. 2019;4(1):122-131.
doi pubmed - Freeman AM, Raman SV, Aggarwal M, Maron DJ, Bhatt DL, Parwani P, Osborne J, et al. Integrating coronary atherosclerosis burden and progression with coronary artery disease risk factors to guide therapeutic decision making. Am J Med. 2023;136(3):260-269.e267.
doi pubmed - Prostate Cancer UK. Transform: A primary cardiovascular disease prevention trial [Internet]. 2024 [cited 2024 Oct 29]. Available from: https://transformtrial.org/.
- Sachdeva A, Cannon CP, Deedwania PC, Labresh KA, Smith SC, Jr., Dai D, Hernandez A, et al. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J. 2009;157(1):111-117 e112.
doi pubmed - Muhlestein JB, Lappe DL, Lima JA, Rosen BD, May HT, Knight S, Bluemke DA, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA. 2014;312(21):2234-2243.
doi pubmed - Serruys PW, Hara H, Garg S, Kawashima H, Norgaard BL, Dweck MR, Bax JJ, et al. Coronary computed tomographic angiography for complete assessment of coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78(7):713-736.
doi pubmed - Mezquita AJV, Biavati F, Falk V, Alkadhi H, Hajhosseiny R, Maurovich-Horvat P, Manka R, et al. Clinical quantitative coronary artery stenosis and coronary atherosclerosis imaging: a Consensus Statement from the Quantitative Cardiovascular Imaging Study Group. Nat Rev Cardiol. 2023;20(10):696-714.
doi pubmed - Williams MC, Earls JP, Hecht H. Quantitative assessment of atherosclerotic plaque, recent progress and current limitations. J Cardiovasc Comput Tomogr. 2022;16(2):124-137.
doi pubmed - Takagi H, Leipsic JA, Indraratna P, Gulsin G, Khasanova E, Tzimas G, Lin FY, et al. Association of tube voltage with plaque composition on coronary CT angiography: results from PARADIGM registry. JACC Cardiovasc Imaging. 2021;14(12):2429-2440.
doi pubmed - Aldana-Bitar J, Golub IS, Moore J, Krishnan S, Verghese D, Manubolu VS, Benzing T, et al. Colchicine and plaque: a focus on atherosclerosis imaging. Prog Cardiovasc Dis. 2024;84:68-75.
doi pubmed - Machado MF, Felix N, Melo PHC, Gauza MM, Calomeni P, Generoso G, Khatri S, et al. Coronary computed tomography angiography versus invasive coronary angiography in stable chest pain: a meta-analysis of randomized controlled trials. Circ Cardiovasc Imaging. 2023;16(11):e015800.
doi pubmed - Nurmohamed NS, Bom MJ, Jukema RA, de Groot RJ, Driessen RS, van Diemen PA, de Winter RW, et al. AI-guided quantitative plaque staging predicts long-term cardiovascular outcomes in patients at risk for atherosclerotic CVD. JACC Cardiovasc Imaging. 2024;17(3):269-280.
doi pubmed - Burch RA, Siddiqui TA, Tou LC, Turner KB, Umair M. The cost effectiveness of coronary CT angiography and the effective utilization of CT-fractional flow reserve in the diagnosis of coronary artery disease. J Cardiovasc Dev Dis. 2023;10(1).
doi pubmed - Drobni ZD, Kolossvary M, Karady J, Jermendy AL, Tarnoki AD, Tarnoki DL, Simon J, et al. Heritability of coronary artery disease: insights from a classical twin study. Circ Cardiovasc Imaging. 2022;15(3):e013348.
doi pubmed - Klarin D, Natarajan P. Clinical utility of polygenic risk scores for coronary artery disease. Nat Rev Cardiol. 2022;19(5):291-301.
doi pubmed - Honigberg MC, Truong B, Khan RR, Xiao B, Bhatta L, Vy HMT, Guerrero RF, et al. Polygenic prediction of preeclampsia and gestational hypertension. Nat Med. 2023;29(6):1540-1549.
doi pubmed - Nakao T, Yu Z, Vlasschaert C, Uddin MM, Lindsay ME, Ellinor PT, Ebert BL, et al. Increased risk of thoracic aortic aneurysms with JAK2 V617F. J Am Coll Cardiol. 2023;81(21):2128-2130.
doi pubmed - Tcheandjieu C, Zhu X, Hilliard AT, Clarke SL, Napolioni V, Ma S, Lee KM, et al. Large-scale genome-wide association study of coronary artery disease in genetically diverse populations. Nat Med. 2022;28(8):1679-1692.
doi pubmed - Aragam KG, Jiang T, Goel A, Kanoni S, Wolford BN, Atri DS, Weeks EM, et al. Discovery and systematic characterization of risk variants and genes for coronary artery disease in over a million participants. Nat Genet. 2022;54(12):1803-1815.
doi pubmed - Khan SS, Post WS, Guo X, Tan J, Zhu F, Bos D, Sedaghati-Khayat B, et al. Coronary artery calcium score and polygenic risk score for the prediction of coronary heart disease events. JAMA. 2023;329(20):1768-1777.
doi pubmed - Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13(5):376-389.
doi pubmed - Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488-2498.
doi pubmed - Fidler TP, Xue C, Yalcinkaya M, Hardaway B, Abramowicz S, Xiao T, Liu W, et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature. 2021;592(7853):296-301.
doi pubmed - Vaiserman A, Krasnienkov D. Telomere length as a marker of biological age: state-of-the-art, open issues, and future perspectives. Front Genet. 2020;11:630186.
doi pubmed - van der Harst P, van der Steege G, de Boer RA, Voors AA, Hall AS, Mulder MJ, van Gilst WH, et al. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49(13):1459-1464.
doi pubmed - Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358(9280):472-473.
doi pubmed - Hoffmann J, Richardson G, Haendeler J, Altschmied J, Andres V, Spyridopoulos I. Telomerase as a therapeutic target in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2021;41(3):1047-1061.
doi pubmed - Maier R, Bawamia B, Bennaceur K, Dunn S, Marsay L, Amoah R, Kasim A, et al. Telomerase activation to reverse immunosenescence in elderly patients with acute coronary syndrome: protocol for a randomized pilot trial. JMIR Res Protoc. 2020;9(9):e19456.
doi pubmed - Abdelrahman KM, Chen MY, Dey AK, Virmani R, Finn AV, Khamis RY, Choi AD, et al. Coronary computed tomography angiography from clinical uses to emerging technologies: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(10):1226-1243.
doi pubmed - Marwan M, Taher MA, El Meniawy K, Awadallah H, Pflederer T, Schuhback A, Ropers D, et al. In vivo CT detection of lipid-rich coronary artery atherosclerotic plaques using quantitative histogram analysis: a head to head comparison with IVUS. Atherosclerosis. 2011;215(1):110-115.
doi pubmed - Williams MC, Kwiecinski J, Doris M, McElhinney P, D'Souza MS, Cadet S, Adamson PD, et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results From the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation. 2020;141(18):1452-1462.
doi pubmed - Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58(8):849-860.
doi pubmed - Schuijf JD, Lima JAC, Boedeker KL, Takagi H, Tanaka R, Yoshioka K, Arbab-Zadeh A. CT imaging with ultra-high-resolution: Opportunities for cardiovascular imaging in clinical practice. J Cardiovasc Comput Tomogr. 2022;16(5):388-396.
doi pubmed - Nieman K, Garcia-Garcia HM, Hideo-Kajita A, Collet C, Dey D, Pugliese F, Weissman G, et al. Standards for quantitative assessments by coronary computed tomography angiography (CCTA): An expert consensus document of the society of cardiovascular computed tomography (SCCT). J Cardiovasc Comput Tomogr. 2024;18(5):429-443.
doi pubmed - Douglas PS, Nanna MG, Kelsey MD, Yow E, Mark DB, Patel MR, Rogers C, et al. Comparison of an initial risk-based testing strategy vs usual testing in stable symptomatic patients with suspected coronary artery disease: the PRECISE randomized clinical trial. JAMA Cardiol. 2023;8(10):904-914.
doi pubmed - Jonas RA, Weerakoon S, Fisher R, Griffin WF, Kumar V, Rahban H, Marques H, et al. Interobserver variability among expert readers quantifying plaque volume and plaque characteristics on coronary CT angiography: a CLARIFY trial sub-study. Clin Imaging. 2022;91:19-25.
doi pubmed - Omori H, Matsuo H, Fujimoto S, Sobue Y, Nozaki Y, Nakazawa G, Takahashi K, et al. Determination of lipid-rich plaques by artificial intelligence-enabled quantitative computed tomography using near-infrared spectroscopy as reference. Atherosclerosis. 2023;386:117363.
doi pubmed - van Rosendael AR, Crabtree T, Bax JJ, Nakanishi R, Mushtaq S, Pontone G, Andreini D, et al. Rationale and design of the CONFIRM2 (Quantitative coronary CT angiography evaluation for evaluation of clinical outcomes: an international, multicenter registry) study. J Cardiovasc Comput Tomogr. 2024;18(1):11-17.
doi pubmed - Nurmohamed NS, Cole JH, Budoff MJ, Karlsberg RP, Gupta H, Sullenberger LE, Quesada CG, et al. Impact of atherosclerosis imaging-quantitative computed tomography on diagnostic certainty, downstream testing, coronary revascularization, and medical therapy: the CERTAIN study. Eur Heart J Cardiovasc Imaging. 2024;25(6):857-866.
doi pubmed - Antonopoulos AS, Sanna F, Sabharwal N, Thomas S, Oikonomou EK, Herdman L, Margaritis M, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9(398).
doi pubmed - Sagris M, Antonopoulos AS, Simantiris S, Oikonomou E, Siasos G, Tsioufis K, Tousoulis D. Pericoronary fat attenuation index-a new imaging biomarker and its diagnostic and prognostic utility: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2022;23(12):e526-e536.
doi pubmed - Almeida S, Pelter M, Shaikh K, Cherukuri L, Birudaraju D, Kim K, Modi J, et al. Feasibility of measuring pericoronary fat from precontrast scans: Effect of iodinated contrast on pericoronary fat attenuation. J Cardiovasc Comput Tomogr. 2020;14(6):490-494.
doi pubmed - Chan K, Wahome E, Tsiachristas A, Antonopoulos AS, Patel P, Lyasheva M, Kingham L, et al. Inflammatory risk and cardiovascular events in patients without obstructive coronary artery disease: the ORFAN multicentre, longitudinal cohort study. Lancet. 2024;403(10444):2606-2618.
doi pubmed - Demircioglu A, Bos D, Demircioglu E, Qaadan S, Glasmachers T, Bruder O, Umutlu L, et al. Deep learning-based scan range optimization can reduce radiation exposure in coronary CT angiography. Eur Radiol. 2024;34(1):411-421.
doi pubmed - Sandler S, Andersson A. Islet implantation into diabetic mice with pancreatic insulitis. Acta Pathol Microbiol Scand A. 1981;89(2):107-112.
doi pubmed - Madaj P, Li D, Nakanishi R, Andreini D, Pontone G, Conte E, O'Rourke Franzcr R, et al. Lower radiation dosing in cardiac CT angiography: the CONVERGE registry. J Nucl Med Technol. 2020;48(1):58-62.
doi pubmed - Bejar N, Tat TT, Kiss DL. RNA therapeutics: the next generation of drugs for cardiovascular diseases. Curr Atheroscler Rep. 2022;24(5):307-321.
doi pubmed - Nazir A, Uwishema O, Shariff S, Franco WXG, El Masri N, Ayele ND, Munyangaju I, et al. A thorough navigation of miRNA's blueprint in crafting cardiovascular fate. Health Sci Rep. 2024;7(11):e70136.
doi pubmed - Tsiachristas A, Chan K, Wahome E, Kearns B, Patel P, Lyasheva M, Syed N, et al. Cost-effectiveness of a novel AI technology to quantify coronary inflammation and cardiovascular risk in patients undergoing routine Coronary Computed Tomography Angiography. Eur Heart J Qual Care Clin Outcomes. 2024.
doi pubmed - Hanneman K, Playford D, Dey D, van Assen M, Mastrodicasa D, Cook TS, Gichoya JW, et al. Value creation through artificial intelligence and cardiovascular imaging: a scientific statement from the American Heart Association. Circulation. 2024;149(6):e296-e311.
doi pubmed - Packard RR, Nakanishi R, Lajoie A, et al. The first clinical use of fractional flow reserve derived from coronary CT angiography (FFR-CT) in the ‘Real World’ predicts standard of care guided revascularization. Circulation. 2015;132(suppl_3):A15042.
- Zeb I, Li D, Nasir K, Malpeso J, Batool A, Flores F, Dailing C, et al. Effect of statin treatment on coronary plaque progression - a serial coronary CT angiography study. Atherosclerosis. 2013;231(2):198-204.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.