| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 3, June 2025, pages 225-237
Validation of Decisions for Percutaneous Coronary Intervention Using Smartphone-Based Electrocardiogram Device Spandan: A Cross-Sectional Observational Study
CB Pandeya, Yogendra Singhb, Shashank Pandeya, Deepak Tomara, Nitin Chandolac, d, Deeksha Agarwalc, Sengar Yashwardhan Pratap Singhc
aDepartment of Cardiology, Lala Lajpat Rai Medical College, Meerut, Uttar Pradesh, India
bDepartment of Cardiology, Max Super Speciality Hospitals, Dehradun, Uttarakhand, India
cResearch and Development, Department of Clinical Research, Sunfox Technologies, Dehradun, Uttarakhand, India
dCorresponding Author: Nitin Chandola, Research and Development, Department of Clinical Research, Sunfox Technologies, Dehradun 248001, India
Manuscript submitted February 1, 2025, accepted March 29, 2025, published online May 7, 2025
Short title: Spandan ECG Device for PCI Decisions
doi: https://doi.org/10.14740/cr2051
| Abstract | ▴Top |
Background: India bears a high burden of acute coronary syndrome, with younger patients and a high prevalence of ST-elevation myocardial infarction (STEMI). Spandan is, therefore, an attractive smartphone-based electrocardiogram (ECG) device that could allow for potentially early diagnosis as well as enabling timely intervention which may even save lives in resource-poor settings. The study aimed to assess the performance and diagnostic capability of the Spandan smartphone-based ECG device in decision-making for percutaneous coronary intervention (PCI) by analyzing the initial ST-segment elevation, which was compared to a 12-lead ECG as the gold standard (BPL Cardiart ECG Machine).
Methods: This was an observational cross-sectional study involving 184 eligible participants with chest pain presenting to the local hospital, in Meerut, Uttar Pradesh, India. The study was conducted for the evaluation of the diagnostic appropriateness of the Spandan ECG device for the detection of ST elevation as compared to standard 12-lead ECGs so that the cardiologists could be more easily guided in their decisions relative to PCI. Patients with the onset of chest pain within or after 120 h and ST elevation above 1 mm in two or more leads were enrolled and patients with dementia, bundle branch block, cardiogenic shock, and ECG artifacts were excluded. The analysis included calculating response characteristics and estimating correlation coefficients and confusion matrix to compare both appraisal methods.
Results: The Spandan device performed with good agreement with the gold standard ECG, particularly in the leads II, III, and AVF, with Pearson correlation coefficients close to 1. The ST elevation in the Spandan device showed no statistical difference compared to the 12-lead ECG. The device exhibited a sensitivity of 94% and a positive predictive value of 94% for ST-elevation detection, thus having supportive evidence for possible usefulness for decision-making in PCI.
Conclusions: ECG findings, such as that of the smartphone-based device (Spandan Pro ECG, a single channel autoswitched ECG machine), demonstrated comparable accuracy with the gold standard 12-lead ECG for the diagnosis of ST elevation and helped in making clinical decisions in patients requiring PCI, especially in resource-limited settings.
Keywords: Acute coronary syndrome; ST-elevation myocardial infarction; Percutaneous coronary intervention; Portable ECG; Spandan ECG
| Introduction | ▴Top |
Acute coronary syndrome (ACS) refers to several conditions that include ST-elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction (NSTEMI), and unstable angina. It is a type of coronary heart disease (CHD), which accounts for almost one-third of the total deaths in individuals above 35 years of age. Some of the forms of CHD are asymptomatic, but ACS is definitely symptomatic [1]. India bears the world’s highest burden of ACS, with patients being younger (average age 56.3 years) and having a higher prevalence of STEMI at over 60.6%, leading to an estimated 3 million STEMI cases annually [2]. STEMI is a clinical syndrome defined by characteristic symptoms of myocardial ischemia in association with persistent electrocardiographic (ECG) ST elevation and subsequent release of biomarkers of myocardial necrosis [3]. Rapid reperfusion therapy remains the cornerstone of treatment for acute STEMI [4, 5]. The sooner it can be instituted, the better the outcome will be [6]. The primary percutaneous coronary intervention (PCI) mechanical reperfusion results in lesser morbidity and mortality than that of fibrinolysis [4, 5, 7, 8].
Conventional practice for STEMI diagnosis via 12-lead ECG can be delayed in resource-limited settings or prehospital logistics due to the need for specialized equipment which will also be equipped by non-professionals. One major limitation to the wider use of primary PCI is the system delay from the first contact with the emergency medical services to balloon inflation, due to a limited number of 24/7 PCI centers and long transport distances. The identification and optimization of prehospital logistics, therefore, become very crucial, along with the reduction of system delay in STEMI patients [9]. Many initiatives have been taken all over the world to overcome this challenge [10-12]. The use of prehospital ECG diagnosis in STEMI has been shown in numerous reports in highly selected patient populations to reduce treatment delay [13-18]. At this point, the emergence of smartphone-based ECG devices offers portability, accessibility, and even cost-effectiveness in early diagnosis and decision-making in acute cardiac events at the point of care.
One such invention is the Spandan Pro smartphone-based ECG device developed by Sunfox Technologies Private Limited, Dehradun, India, which uses a smartphone platform to provide accurate ECG readings (Fig. 1). The device has proven to be a convenient and reliable diagnostic tool for STEMI, potentially revolutionizing the management of ACS. To assure its clinical utility and wider implementation, however, thorough validation of its effectiveness in guiding treatment decisions is necessary, especially concerning PCI.
 Click for large image | Figure 1. Spandan portable ECG developed by Sunfox Technologies Pvt. Ltd. A smartphone-based portable ECG device that is capable of taking 12-lead ECGs by using derived ECG methods to evaluate STEMI/NSTEMI. ECG: electrocardiogram; NSTEMI: non-ST-elevation myocardial infarction; STEMI: ST-elevation myocardial infarction. |
Several studies have investigated the effectiveness of smartphone-based ECG devices in the facilitation of better outcomes for patients with ACS, particularly STEMI. These devices have the potential to solve the challenge posed by conventional methods, such as the difficulty with current approaches to delays in diagnosis and treatment commencement, especially in resource-limited settings.
The present cross-sectional observational study aimed to address this critical gap in evidence by assessing the effectiveness of decisions regarding PCI or coronary angioplasty using the Spandan device. Additionally, the study sought to determine the utility of initial ST-segment elevation in guiding the selection of the treatment strategy. The primary objective of this study was to provide robust evidence for the incorporation of smartphone ECG-based technology in the management of STEMI by better investigating the diagnostic accuracy of the Spandan device compared with the gold standard 12-lead ECG.
Therefore, findings from this study hold significant clinical practice implications, potentially making decisions to streamline the diagnosis of STEMI and treatment decision-making, particularly in resource-limited settings. In addition, this study will add insights into the role of initial ST-segment elevation in the treatment strategy selection for the development process for refining evidence-based guidelines on the management of STEMI.
This study aimed to improve patient outcomes and optimize resource utilization in cardiovascular care by offering insightful information about the evolving field of STEMI diagnosis and treatment through meticulous design, significant data collection, and rigorous statistical analysis.
| Materials and Methods | ▴Top |
Study design
This study was designed with meticulous care: as a single-blinded, observational, and cross-sectional design. It aimed to evaluate the efficacy of decisions regarding PCI or coronary angioplasty using the smartphone-based ECG device Spandan Pro. Additionally, the study aimed to validate the utility of initial ST-segment elevation in determining the treatment strategy for patients presenting with ACS.
Settings
The data were collected at the local hospital, in Meerut, Uttar Pradesh, India, from May 16 to November 24, 2023, involving the emergency department and the ECG room to ensure proper evaluation and management of all the patients who report symptoms and signs of ACS.
Participants
The study cohort, which includes 200 individuals who are 20 years of age or older, was chosen from the hospital’s emergency and ECG rooms. Chest pain within or developing after 120 h and ST elevation greater than 1 mm in two or more leads on the initial ECG were used to select participants. The following conditions must be met to be excluded: patients with dementia, complete bundle branch block, cardiogenic shock, higher-than-normal risk of bleeding, baseline wandering, and artifacts in ECG readings. A total of 184 eligible participants were included in the study once the exclusion criteria were applied.
Among 184 patients, the patient’s demographic and clinical data were divided into patients for whom PCI was recommended and those for whom the procedure was not recommended. Key variables included gender, diabetic, smoking, and coronary artery disease (CAD) (Table 1).
 Click to view | Table 1. Baseline Characteristics of Patients |
Ethical considerations
This study adhered to ethical guidelines, ensuring patient confidentiality, safety, and well-being. It was a prospective research study approved by the Institutional Ethics Committee of LLRM Medical College, Meerut (approval no. SC-1/2025/2170). All procedures were conducted in accordance with the ethical principles outlined in the Declaration of Helsinki.
Supervision and oversight
All tests and procedures were carried out under the direct observation of a cardiologist to ensure that they were carried out by standard protocols and that there was minimal potential for bias as possible. The integrity of this study is maintained because of the rigorous and attentive supervision that ensures the high accuracy and reliability of the data collected.
Reference standard
The diagnostic accuracy of the Spandan 12-lead ECG was compared to that of the conventional gold standard 12-lead ECG. Both ECG reports were interpreted by a cardiologist, and the interpretations were subsequently compared to validate the Spandan device’s alignment with PCI recommendations. Treatment decisions for participants were based solely on the cardiologist’s interpretation of the conventional gold standard 12-lead ECG. While treatment decisions could also be informed by the Spandan device, no direct interventions or treatment decisions were made based on its findings in this study.
Data collection
Upon the presentation of chest pain, each subject was assessed and documented wholly with informed consent and a medical history recorded in detail in the case report form (CRF). The first ECG recordings were obtained with both the gold standard 12-lead ECG and the smartphone-based ECG device Spandan Pro. Based on the interpretation of the gold standard ECG, treatment and testing plans were made, and the ECG obtained via Spandan was interpreted separately by the cardiologist for validation of accuracy.
Timing considerations
The potential biases in this comparative study were minimized by ensuring that the time interval between the generation of the Spandan ECG report and the conventional gold standard 12-lead ECG report did not exceed 5 to 6 h. This time restriction was put in place to ensure uniformity in the comparison by accounting for cases in which the gold standard ECG was conducted outside of regular working hours (late night to early morning) or in emergency department cases where the physician’s primary focus is on stabilizing the serious patient, which may delay the recording of the Spandan ECG. Temporal separation ensured that the assessment of diagnostic accuracy was unbiased and rigorous, reducing the risk of any bias by interpretation.
In some cases, the time discrepancy of more than 30 min to 1 h between the two reports may contribute to the variation, as the patient’s cardiac condition could be in a phase of evolving during this period. In this study, there was a case with a time discrepancy of 5 h and 19 min between both reports. The ST elevation was less pronounced in the Spandan ECG over time. Specifically, in the gold standard, the ST elevation measured 4 mm in V1, 5 mm in V2, 7 mm in V3, 7 mm in V4, and 2 mm in V5, respectively (Fig. 2a). Conversely, in Spandan, the ST elevation measured 1 mm in V1, 2 mm in V2, 3 mm in V3, and 2 mm in V4 (Fig. 2b).
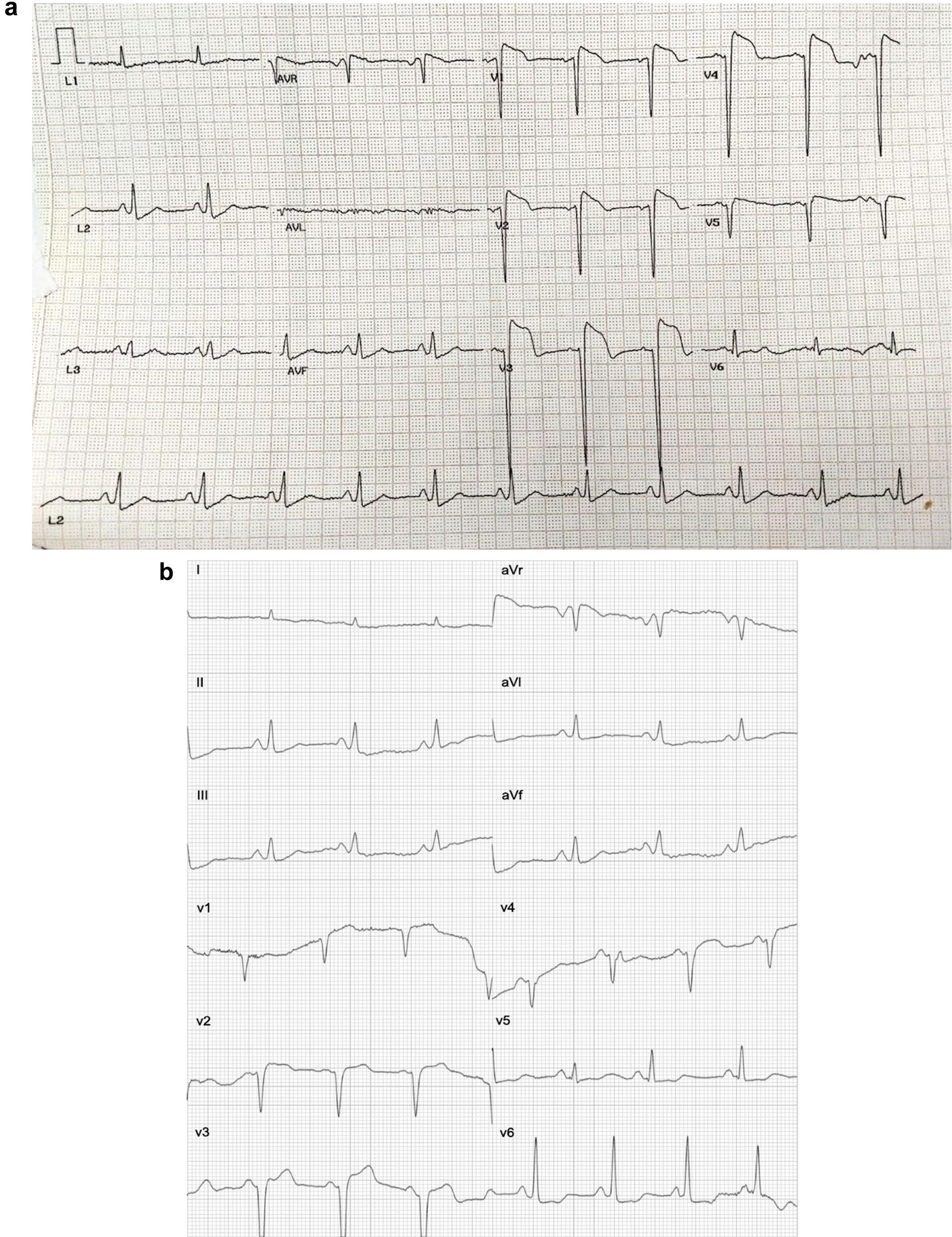 Click for large image | Figure 2. (a) A gold standard ECG test report. The patient’s report indicates ST-elevation, with the test performed on November 24, 2023 at 12:05 pm. (b) Spandan ECG test report. The patient’s report indicates ST-elevation, with the test performed on November 24, 2023 at 4:24 pm. ECG: electrocardiogram. |
Statistical analysis
The evaluation of the diagnostic accuracy of both devices, Spandan and gold standards, to measure the ST elevation and based on it to recommend PCI, was conducted by calculating standard deviation, t-test, confusion matrix, and the correlation coefficient, respectively. The data were analyzed using appropriate software.
Data privacy, security, confidentiality, and storage
The hospital ensures that it will never share data with any department, company, or third party since the safety of patient’s data and its privacy are our top concerns. The data will be treated according to very stringent data handling protocols. All data will be anonymized at the point of collection and archived securely on a two-step authenticated local server of the hospital, with backup storage being maintained through HIPPA-compliant cloud storage and Spreadsheets and also printed sheets stored in the hospital. Access to the data will be restricted to authorized personnel from the hospital. No personally identifiable information will be attached to the analyzed data. In addition, all data transfers will use secure, encrypted channels to maintain the integrity and confidentiality of data.
Archival
Scanned ECG reports and all study-related documents were archived in cloud storage to ensure transparency, facilitate future reference, and enable the scrutiny and verification of study findings.
Spandan Pro ECG device recording system
The Spandan Pro ECG is a 12-lead smartphone-based ECG device designed for real-time cardiac monitoring with a compact and portable setup. It functions similarly to a gold standard 12-lead ECG by recording electrical activity through limb and chest electrodes following Goldberger lead placement. Apply the RA electrode on the right forearm or wrist. LA on the left forearm or wrist. While LL/F and RL/N electrodes were placed on the left and right legs, respectively. The placement of chest electrodes includes: C1 (red), at the right margin of the fourth intercostal space on the sternum, C2 (yellow) at the same level left margin of the sternum, C3 (green) at an equal distance to C2 from the C4, C4 (brown) on the fifth intercostal space along the mid-clavicular line, C5 (black) is placed on the fifth intercostal space between equal distances of C4 and C6, and C6 (purple) at the fifth intercostal space along the mid-axillary line. Unlike traditional hospital-based ECG machines, which are bulky and require battery, Spandan Pro is seamlessly connected to the smartphone via a micro-USB cable, enabling easily accessible and machine-driven ECG interpretation.
The test was then started through the smartphone application; however, during the test, the patient should be ensured to remain still during the procedure. The ECG report generated by the device was later analyzed for clinical interpretation. In this clinical trial, the Goldberg system was used for electrode placement.
Spandan device algorithm for detecting STEMI
A description of the algorithms utilized by the device for STEMI detection, as outlined in Figure 3, was applied for analysis.
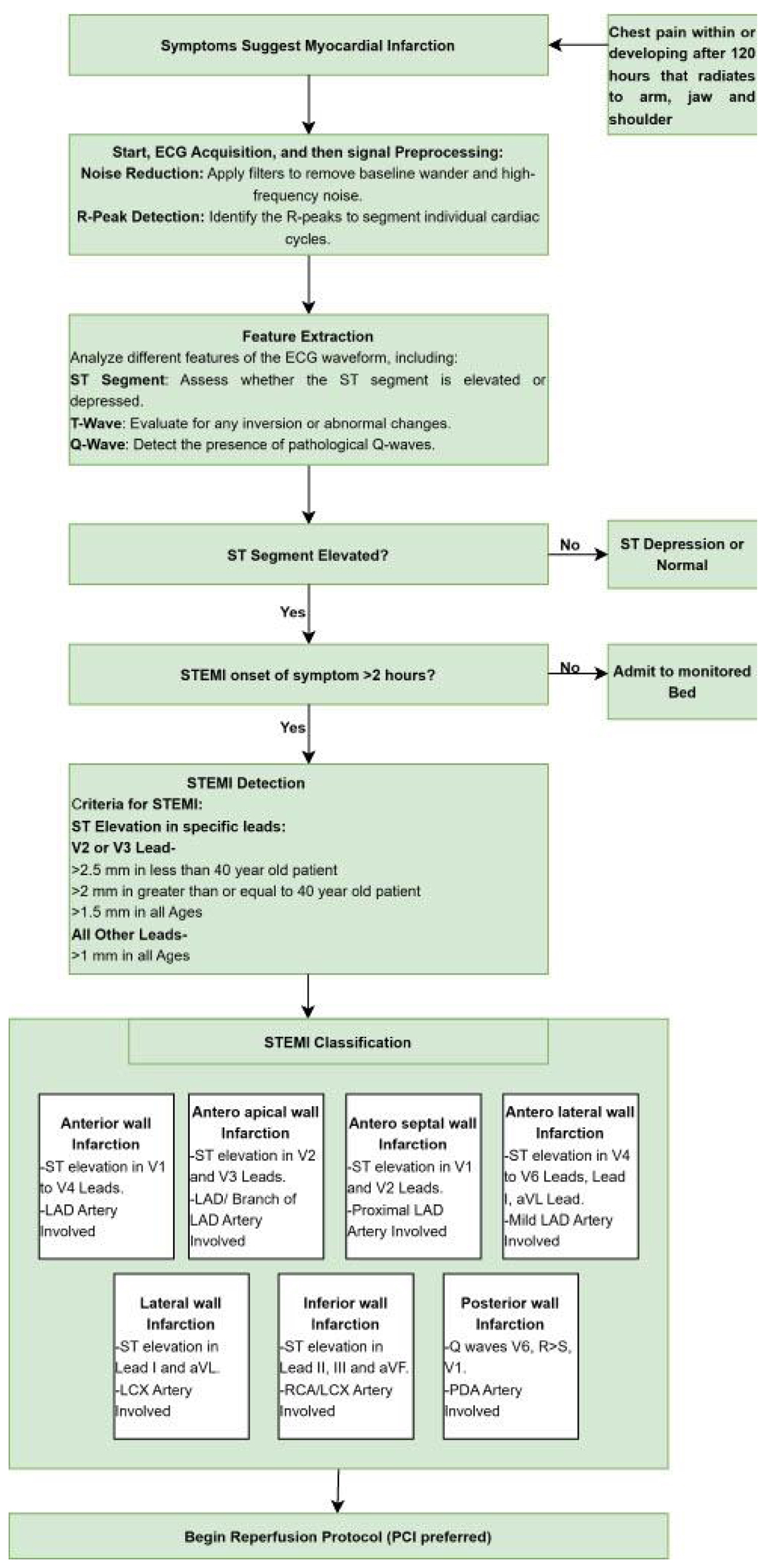 Click for large image | Figure 3. Spandan ECG device algorithm for detecting STEMI. ECG: electrocardiogram. |
| Results | ▴Top |
In this study, 200 patient records were evaluated. However, 16 cases were discarded in the database because of baseline wandering, and the remaining 184 cases were further included for analysis in the cohort. Out of 184 cases considered for analysis, 96 patients developed chest pain within 120 h of follow-up, and 88 patients reported the development of chest pain beyond this period. Of these, 55 had some ST-segment elevation at the time of or any time after the chest pain and within or after 120 h of the onset of symptoms, thus falling within the recommended timeframe for PCI. The remaining 129 did not fall under the PCI recommendation based on ST elevation and timing of symptoms (Fig. 4). Of 55 patients who fit the criteria for PCI, 33 showed up to the hospital with chest pain within the recommended time window of 120 h, while 22 were brought in after this period with chest pain.
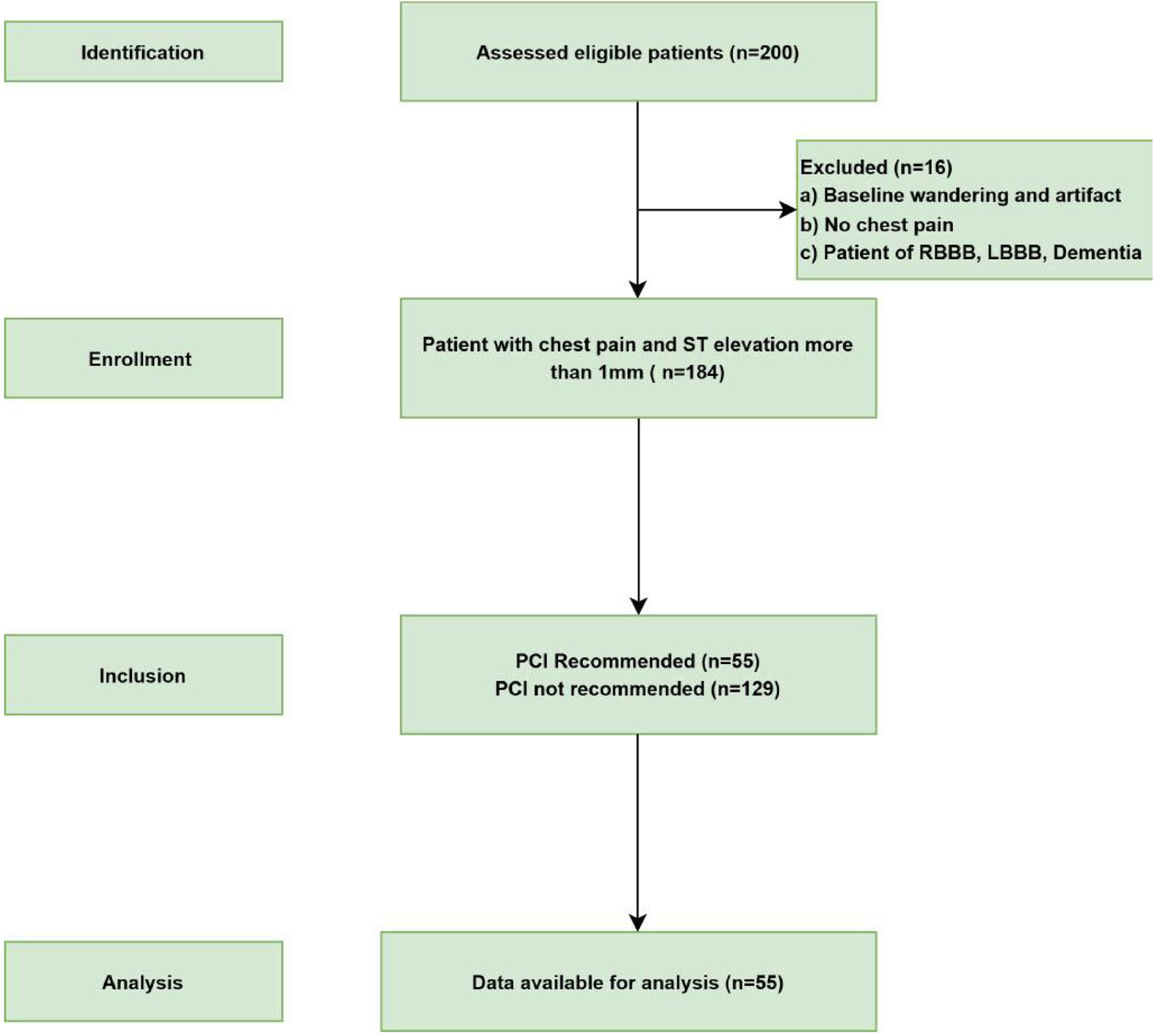 Click for large image | Figure 4. STORBE flow chart of the study. Flow diagram for the inclusion of patients in this study from May 16, 2023 to November 24, 2023 according to the Strengthening the Reporting of Observational Studies in Epidemiology (STORBE) studies. |
The testing for the accuracy of both devices, the gold standard and the Spandan Pro, was based on their recommendations for PCI against the doctor’s diagnosis and the PCI recommendations. Surprisingly, both devices are 100% concordant with the PCI recommendations, which indicates a constant agreement between the device recommendations and the clinically indicated guidelines.
Both reports (Figs. 5 and 6) refer to the same patient who was diagnosed with acute anterolateral MI. The patient was reported to the hospital after 120 h of onset of chest pain. Both reports showed anterior, septal, and lateral leads of ST elevation. Therefore, the recommendation for this patient was to undergo PCI.
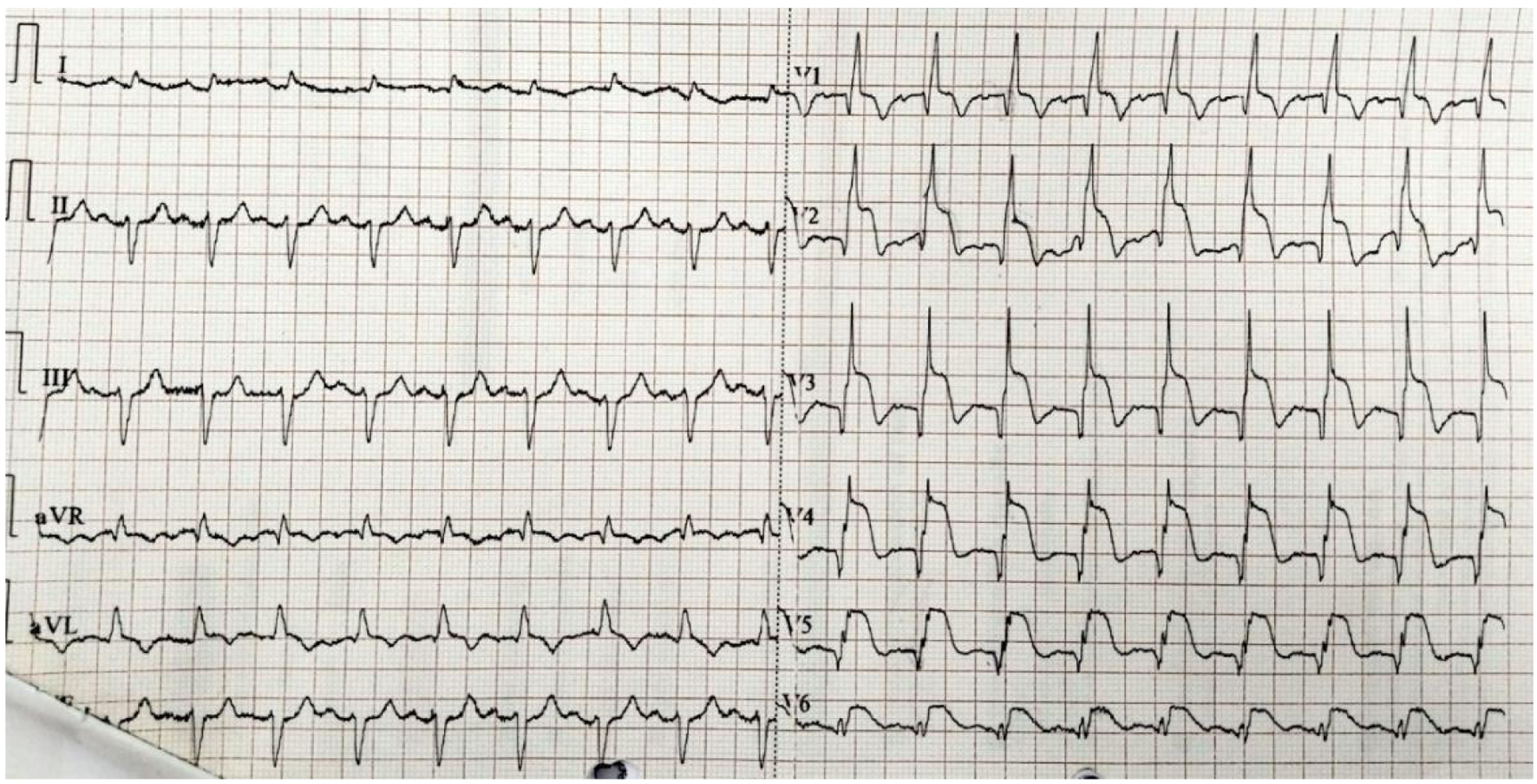 Click for large image | Figure 5. A gold standard ECG test report. The patient’s report indicates anterolateral MI and the test was performed on November 20, 2023. ECG: electrocardiogram; MI: myocardial infarction.Spandan ECG test report. The patient’s report indicates anterolateral MI and the test was performed on November 20, 2023. ECG: electrocardiogram; MI: myocardial infarction. |
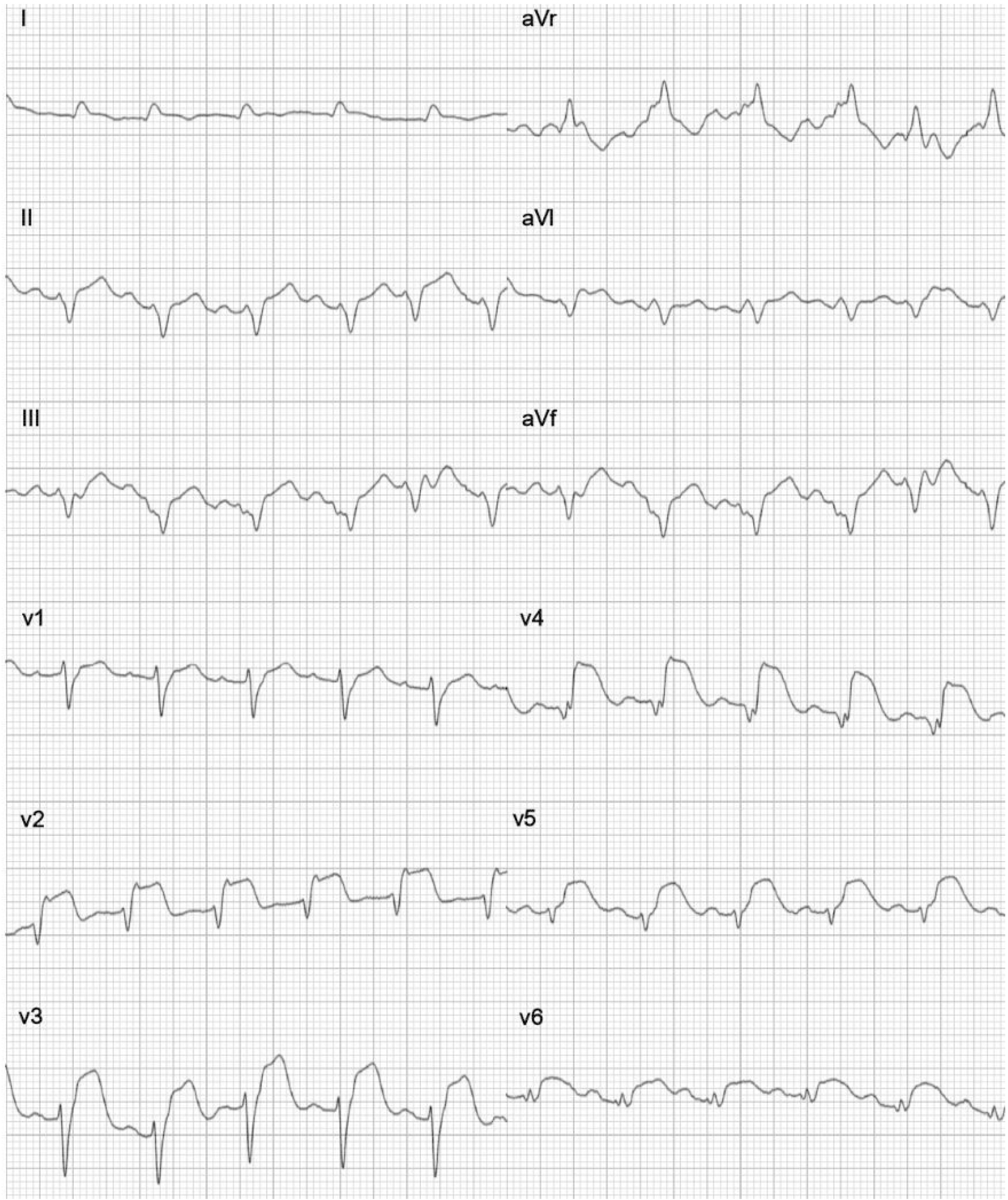 Click for large image | Figure 6. Spandan ECG test report. The patient’s report indicates anterolateral MI and the test was performed on November 20, 2023. ECG: electrocardiogram; MI: myocardial infarction. |
Table 2 allocates STEMI and NSTEMI diagnoses into PCI-recommended cases which outlines the spread of MI and ischemia in various parts of the heart. It comprises 16 cases of anterior wall MI, one anterolateral MI, 33 anteroseptal MI, seven inferior wall MI, six inferolateral MI, and one lateral wall MI. Ischemic cases include six cases of inferior-lateral ischemia, three cases of anterior-septum ischemia, one case of each for the anterior wall, inferior wall, and lateral wall ischemia, and four cases of unspecified ischemia. This provides a comprehensive view of the infarction and ischemia locations in patients recommended for PCI.
 Click to view | Table 2. Classification of STEMI/NSTEMI Diagnosis in PCI Recommended Cases |
Moreover, the ST-elevation measurements for leads V1-V6 and leads I, II, III, AVF, and AVL were recorded on both devices against all the cases that met the recommendation criteria of PCI. Such comprehensive assessment will enable a detailed analysis of ST-elevation patterns and their correlation with the recommendations under PCI, hence helping to validate the efficacy of the Spandan device in guiding the ACS treatment decision.
In the present study, the standard deviations of differences in ST-elevation measurement between the Spandan device and the gold standard method have been calculated for various ECG leads: V1-V6, I, II, III, AVL, and AVF. The standard deviations were observed from a minimum of 0.067 mm in lead III to a maximum of 0.812 mm in lead V3, thereby indicating that Spandan’s agreement with the gold standard varied from lead to lead. This is as indicated in Table 3. Leads V2, V3, and V4 recorded the highest standard deviations, thus the most spread-out dispersion of the difference in measures on ST elevation between the two methods. On the other hand, leads I, II, AVL, III, and AVF had the lowest standard deviation measures, showing that there is more consistency in measurement differences. This variability in agreement across leads underlines the requirement for further study of factors influencing the consistency and performance of the Spandan device with particular reference to those leads that exhibit higher variability (Table 3).
 Click to view | Table 3. Standard Deviation of Difference in ST-Elevation of Spandan ECG and Gold Standard ECG |
Table 4 presents the P-values obtained from a statistical test (likely a t-test) comparing the ST-elevation measurements between the smartphone-based ECG device Spandan and the gold standard method across various leads (V1, V2, V3, etc.).
 Click to view | Table 4. P-Value of All the Leads of the ST-Elevation Obtained From the Spandan ECG and Gold Standard ECG |
All the P-values in Table 4 are ranging from 0.725 to 1. In no lead was the null hypothesis rejected which means that it shows a minimum difference between Spandan and the gold standard ECG. There were minimum differences in the ST-elevation measurements obtained by the two devices that could not have occurred by chance.
Table 5 expresses the Pearson correlation coefficients computed to evaluate the relationships between ST-elevation measurements obtained from Spandan and the gold standard method across all leads such as V1, V2, V3, etc.
 Click to view | Table 5. Pearson Correlation of All Leads of the ST-Elevation Obtained From the Spandan ECG and Gold Standard ECG |
This is substantiated further by high correlation coefficients in Table 5 related to most leads, which implies a strong positive linear relationship of measurements obtained from Spandan regarding a gold standard method. Even more important are leads II, III, and AVF, which have the highest correlation coefficients close to 1, indicating that the signal is almost perfectly positive and linear between the measurements of the devices.
For example, lead I shows a correlation coefficient of 1, meaning that there is an exact positive linear relationship between measurements from both devices. For this reason, amongst the chest leads, the correlation coefficient of measurements between Spandan and the gold standard is the highest in lead V5.
The concluding part is that the results appraise a strong positive linear relationship between the measurements obtained from Spandan and the gold standard across most leads, where correlation coefficients are turning out to be very high in some leads, showing almost perfect agreement across the devices. The findings suggest that they are comparable and very reliable in the ST-elevation measurement as a smartphone-based device for ECG.
Table 6 presents a comparative analysis of the diagnostic performance of the Spandan ECG and the gold standard 12-lead ECG in detecting ST elevation. The key performance metrics include true positive (TP), true negative (TN), false positive (FP), false negative (FN), sensitivity, and positive predictive value (PPV).
 Click to view | Table 6. Comparative Diagnostic Performance of Spandan ECG and Gold Standard 12-Lead ECG for ST-Elevation Detection |
Confounding factors
Analysis of demographic variability (age, gender, and comorbidity) and its influence on the results are described in Table 7.
 Click to view | Table 7. Confounding Factor - Variability in Patient Demographics |
| Discussion | ▴Top |
This study evaluated the accuracy and reliability of the Spandan device in measuring ST elevation compared to the gold standard ECG method, specifically focusing on its potential application in guiding PCI decisions in patients presenting with chest pain.
The Spandan ECG can be used to rule in which patients need rapid detection and transport to a PCI center, without having many false alarms. This could be of great importance to reduce time to treatment in a patient with atypical complaints.
A significant strength of our study is the large sample size of 184 patients, providing a robust dataset for analysis. Of the patients, 129 did not meet the criteria for PCI based on ST elevation and symptom timing. Among the 55 who met PCI criteria, 33 arrived at the hospital within the crucial 120-h window, while 22 presented after this period, potentially reducing the effectiveness of PCI. Additionally, the inclusion of patients with varying degrees of ST elevation and time delays in presentation strengthens the generalizability of our findings. Furthermore, the comprehensive evaluation of ST-elevation measurements across multiple leads enhances the understanding of Spandan’s performance in different anatomical regions of the heart.
Principal findings
The Spandan device demonstrated a strong positive linear relationship with the gold standard ECG across most leads, as indicated by high Pearson correlation coefficients. In particular, lead I showed perfect agreement and leads II, III, and AVF showed correlation coefficients close to 1, suggesting almost perfect agreement.
The t-tests conducted across various leads indicated that there was no statistically significant difference between the ST-elevation measurements obtained from Spandan and the gold standard. All P-values were above the classical threshold of 0.05, suggesting that any observed differences might be due to random variation rather than systematic error.
The Spandan ECG demonstrated a sensitivity of 94%, slightly higher than the gold standard ECG (92%), indicating that it effectively detects true ST-elevation cases with a low FN rate. The PPV of Spandan ECG (94%) is slightly lower than the gold standard ECG (96%), meaning that when the device detects ST elevation, there is a high probability that the diagnosis is correct.
Comparison with existing literature
In this study, 184 patients were diagnosed with STEMI, and of them, 73.9% were males, while the most affected age group was 41 to 60. It was strongly associated with diabetes and the severity of STEMI. Diabetes has a significant association with worse clinical outcomes in STEMI patients. These results are in agreement with previous studies, which also reported a significant association between diabetes and adverse outcomes in STEMI patients [19, 20]. Moreover, 44% of STEMI patients in this study were smokers which highlights the strong association between smoking and STEMI severity. This observation is in keeping with the previous studies [19, 21].
Several studies have explored the accuracy of smartphone-based ECG devices in detecting ST elevation. For instance, a study by Muhlestein et al (2015) reported that a smartphone ECG device demonstrated excellent correlation with the gold standard 12-lead ECG in all patients. Four out of six tracings were judged to meet STEMI criteria [22]. Similarly, our study found a strong positive correlation between the Spandan device and the gold standard, especially in leads II, III, and AVF, supporting these findings.
The earlier study by Redfors et al (2021) has already established that symptom-to-door time is a very strong predictor of outcomes in patients presenting with STEMI [23]. In this study, the availability of a Spandan smartphone-based ECG at the level of residence or institutional and departmental level where a standard 12-lead ECG is not available can significantly expedite the decision-making for PCI and can result in an improvement of outcomes in patients.
Studies by van der Ende et al (2020) have shown that 15-30% of patients with acute MI do not recognize their symptoms and either never contact emergency services or do so only after the optimal window for early PCI (< 12 h) has passed [24]. Our study demonstrated the value of the Spandan smartphone-based ECG in detecting ST deviations in patients with intermittent angina pectoris or atypical symptoms, highlighting its potential to improve early detection and timely intervention.
Our experience with the Spandan ECG device has demonstrated its ability to detect nonspecific ST-T-wave changes and aspecific ST-segment depression, which are commonly observed in individuals with a narrow antero-posterior thoracic diameter, concave-shaped chest wall conformation, and/or mitral valve prolapse (MVP). While our study primarily focused on ST elevation, we recognize the importance of distinguishing nonischemic ST-T abnormalities and have addressed this aspect. A previous study by Digeos-Hasnier et al has shown that MVP patients often exhibit prolonged QT intervals and an increased nocturnal QT/RR slope, indicating altered ventricular repolarization and a potential mechanism for arrhythmic risk [25]. Given that the Spandan ECG effectively identifies repolarization abnormalities, it may aid in detecting subtle electrophysiological changes that contribute to arrhythmias in MVP.
Limitations
This analysis could be attributed to Spandan’s lower operational efficiency, as it functions up to 2.5 V compared to the gold standard’s 5 V. The reduced voltage range likely impacts its ability to capture higher amplitudes of electrical activity, resulting in diminished accuracy in detecting significant abnormalities. In most cases, 2.5 V is generally sufficient to capture the necessary amplitudes of electrical activity. Additionally, in some cases, the time discrepancy of more than 30 min to 1 h between the two reports may contribute to the variation, as the patient’s cardiac condition could be in a phase of evolving during this period.
In summary, the approaches to researching and adopting the technologies should deliberately address the barriers of current applicability intra-procedurally to further build on the potential of ECG using smart devices into PCI decision support. It is therefore clear that a smartphone-based ECG has great potential for value in decision support simply due to its portable, convenient, and real-time observability.
This study is limited by the small number of patients included, which may affect the analysis and generalizability of our findings. A larger sample size would provide better conclusions and allow for a more comprehensive evaluation of variability across different patient populations. Future studies with expanded cohorts are needed to further validate our findings.
This study lacks the validation to determine whether ECG findings align consistently with angiographic reports in diagnosing STEMI. Further research is needed to establish whether STEMI decisions can be reliably made directly from ECG reports, ensuring accuracy and improving clinical decision-making.
A non-inferiority analysis was not feasible as only ST-elevation patients were included, resulting in a TN count of zero. Key metrics like specificity and receiver operating characteristic (ROC) curves could not be calculated. Instead, a t-test assessed significant differences in ST-segment elevation between Spandan and gold standard ECGs. The study focuses on ECG-based PCI decisions, not angioplasty outcomes or phlebitis involvement. Further studies should evaluate angioplasty outcomes, long-term prognosis, and broader clinical applications.
Prehospital ECG reducing STEMI mortality
Role of prehospital ECG in STEMI management
Prehospital ECG (Spandan Pro) enables early STEMI diagnosis, pre-alerts PCI-capable hospitals, reduces door-to-balloon time by directing patients appropriately, and improves outcomes by minimizing myocardial damage, short-term mortality, and complications through timely revascularization.
Optimizing prehospital ECG implementation
A 12-lead ECG is essential for accurate STEMI diagnosis. A 12-lead smartphone-based portable device (Spandan Pro) can provide high-quality readings for early detection. It also provides real-time data transmission for confirmation of STEMI by the ECG experts.
Reducing time to PCI
Direct transfer to PCI centers, prehospital ECG transmission, and reducing first medical contact (FMC)-to-balloon time streamline care, enabling faster and more effective PCI.
Conclusion
This study critically assessed the Spandan smartphone-based ECG device for measuring ST elevation, as against the gold standard of 12-lead ECG, in terms of accuracy and reliability, with particular interest paid to its possible role in informing decisions for PCI. The results indicate that across several leads, Spandan has shown a strong positive correlation with the gold standard ECG in many leads; most of them fall within leads II, III, and AVF range, which is an almost perfect agreement. The t-test was presented to indicate that the differences in ST elevation between the devices do not suggest any statistical significance; hence, the variation was probably produced by chance and not by systematic fault. The research records the potential of the Spandan device as an important tool for PCI decision and support, especially in conditions when restructuring needs durations to be short for an accurate diagnosis. However, at the same time, the study emphasizes more research and validation that will further add to its reliability and generalization into clinical practice. On the whole, the Spandan device is promising and is likely to improve outcomes by the ability to prompt well-timed intervention in ACS cases. Additionally, it can be used in prehospital emergency and before sending the patient, medical centers can be informed to reduce the delay in transferring the patient to the Cat Lab unit.
Acknowledgments
The authors are grateful to Department of Cardiology, in Lala Lajpat Rai Medical College, Meerut, Uttar Pradesh, India, and Sunfox Technologies Private Limited, Dehradun, India.
Financial Disclosure
The study was funded exclusively by Sunfox Technologies Pvt. Ltd.
Conflict of Interest
The authors affirm that they have no conflict of interest that could compromise the objectivity, integrity, or impartiality of this research.
Informed Consent
Before being included in the study, each participant provided their informed consent. Both verbally and in writing, participants provided comprehensive information on the purpose, procedures, potential risks, and benefits associated with the study. Consent forms were designed to be easily understandable and included statements ensuring participants’ right to withdraw from the study at any time without any consequences to their medical care. Signed consent forms were collected and securely stored by ethical guidelines.
Author Contributions
Conception: CB Pandey, Yogendra Singh, and Shashank Pandey; Design: CB Pandey, Yogendra Singh, and Deepak Tomar; Supervision: Shashank Pandey, CB Pandey, and Deepak Tomar; Resource: Nitin Chandola, Deeksha Agarwal, and Sengar Yashwardhan Pratap Singh; Materials: Nitin Chandola, Deeksha Agarwal, and Sengar Yashwardhan Pratap Singh; Data collection and/or processing: Nitin Chandola, Deeksha Agarwal, and Shashank Pandey; Analysis and/or interpretation: Shashank Pandey, Nitin Chandola, and Deeksha Agarwal; Literature review: Nitin Chandola, Deeksha Agarwal, and Sengar Yashwardhan Pratap Singh; Writer: Nitin Chandola, Deeksha Agarwal, and Sengar Yashwardhan Pratap Singh; Critical review: CB Pandey, Nitin Chandola, and Yogendra Singh.
Data Availability
The database analyzed in the current study can be obtained from the corresponding author upon reasonable request.
Abbreviations
ACS: acute coronary syndrome; CAD: coronary artery disease; ECG: electrocardiogram; FN: false negative; FP: false positive; LBBB: left bundle branch block; MI: myocardial infarction; MVP: mitral valve prolapse; NSTEMI: non-ST-elevation myocardial infarction; PCI: percutaneous coronary intervention; PPV: positive predictive value; RBBB: right bundle branch block; STEMI: ST-elevation myocardial infarction; TN: true negative; TP: true positive
| References | ▴Top |
- Singh A, Museedi AS, Grossman SA. Acute coronary syndrome. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Alexander T, Mullasari AS, Kaifoszova Z, Khot UN, Nallamothu B, Ramana RG, Sharma M, et al. Framework for a National STEMI Program: consensus document developed by STEMI INDIA, Cardiological Society of India and Association Physicians of India. Indian Heart J. 2015;67(5):497-502.
doi pubmed - Hwang C, Levis JT. ECG diagnosis: ST-elevation myocardial infarction. Perm J. 2014;18(2):e133.
doi pubmed - Canadian Cardiovascular Society; American Academy of Family Physicians; American College of Cardiology, Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51(2):210-247.
doi pubmed - Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29(23):2909-2945.
doi pubmed - De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109(10):1223-1225.
doi pubmed - Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361(9351):13-20.
doi pubmed - Boersma E, Primary Coronary Angioplasty vs. Thrombolysis G. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J. 2006;27(7):779-788.
doi pubmed - Terkelsen CJ, Sorensen JT, Nielsen TT. Is there any time left for primary percutaneous coronary intervention according to the 2007 updated American College of Cardiology/American Heart Association ST-segment elevation myocardial infarction guidelines and the D2B alliance? J Am Coll Cardiol. 2008;52(15):1211-1215.
doi pubmed - Knot J, Widimsky P, Wijns W, Stenestrand U, Kristensen SD, Van THA, Weidinger F, et al. How to set up an effective national primary angioplasty network: lessons learned from five European countries. EuroIntervention. 2009;5(3):299-309.
pubmed - Widimsky P, Wijns W, Fajadet J, de Belder M, Knot J, Aaberge L, Andrikopoulos G, et al. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur Heart J. 2010;31(8):943-957.
doi pubmed - Jacobs AK, Antman EM, Faxon DP, Gregory T, Solis P. Development of systems of care for ST-elevation myocardial infarction patients: executive summary. Circulation. 2007;116(2):217-230.
doi pubmed - Grim P, Feldman T, Martin M, Donovan R, Nevins V, Childers RW. Cellular telephone transmission of 12-lead electrocardiograms from ambulance to hospital. Am J Cardiol. 1987;60(8):715-720.
doi pubmed - Aufderheide TP, Hendley GE, Thakur RK, Mateer JR, Stueven HA, Olson DW, Hargarten KM, et al. The diagnostic impact of prehospital 12-lead electrocardiography. Ann Emerg Med. 1990;19(11):1280-1287.
doi pubmed - Kudenchuk PJ, Maynard C, Cobb LA, Wirkus M, Martin JS, Kennedy JW, Weaver WD. Utility of the prehospital electrocardiogram in diagnosing acute coronary syndromes: the Myocardial Infarction Triage and Intervention (MITI) Project. J Am Coll Cardiol. 1998;32(1):17-27.
doi pubmed - Terkelsen CJ, Lassen JF, Norgaard BL, Gerdes JC, Poulsen SH, Bendix K, Ankersen JP, et al. Reduction of treatment delay in patients with ST-elevation myocardial infarction: impact of pre-hospital diagnosis and direct referral to primary percutanous coronary intervention. Eur Heart J. 2005;26(8):770-777.
doi pubmed - Le May MR, So DY, Dionne R, Glover CA, Froeschl MP, Wells GA, Davies RF, et al. A citywide protocol for primary PCI in ST-segment elevation myocardial infarction. N Engl J Med. 2008;358(3):231-240.
doi pubmed - Pedersen SH, Galatius S, Hansen PR, Mogelvang R, Abildstrom SZ, Sorensen R, Davidsen U, et al. Field triage reduces treatment delay and improves long-term clinical outcome in patients with acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2009;54(24):2296-2302.
doi pubmed - Jensen LO, Maeng M, Thayssen P, Tilsted HH, Terkelsen CJ, Kaltoft A, Lassen JF, et al. Influence of diabetes mellitus on clinical outcomes following primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2012;109(5):629-635.
doi pubmed - Wong MYZ, Yap JJL, Chih HJ, Yan BPY, Fong AYY, Beltrame JF, Wijaya IP, et al. Regional differences in percutaneous coronary intervention outcomes in STEMI patients with diabetes: The Asia-Pacific evaluation of cardiovascular therapies (ASPECT) collaboration. Int J Cardiol. 2023;371:84-91.
doi pubmed - Redfors B, Furer A, Selker HP, Thiele H, Patel MR, Chen S, Udelson JE, et al. Effect of smoking on outcomes of primary PCI in patients with STEMI. J Am Coll Cardiol. 2020;75(15):1743-1754.
doi pubmed - Muhlestein JB, Le V, Albert D, Moreno FL, Anderson JL, Yanowitz F, Vranian RB, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS Pilot Study. J Electrocardiol. 2015;48(2):249-259.
doi pubmed - Redfors B, Mohebi R, Giustino G, Chen S, Selker HP, Thiele H, Patel MR, et al. Time delay, infarct size, and microvascular obstruction after primary percutaneous coronary intervention for ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2021;14(2):e009879.
doi pubmed - van der Ende MY, Juarez-Orozco LE, Waardenburg I, Lipsic E, Schurer RAJ, van der Werf HW, Benjamin EJ, et al. Sex-based differences in unrecognized myocardial infarction. J Am Heart Assoc. 2020;9(13):e015519.
doi pubmed - Digeos-Hasnier S, Copie X, Paziaud O, Abergel E, Guize L, Diebold B, Jeunemaitre X, et al. Abnormalities of ventricular repolarization in mitral valve prolapse. Ann Noninvasive Electrocardiol. 2005;10(3):297-304.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.