| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 000, Number 000, June 2025, pages 000-000
Effect of Angiotensin Receptor-Neprilysin Inhibitor in Patients With Heart Failure: A Real-World Study
Hiroko Mitsudaa, Yuhei Shigaa, Yasunori Suematsua, Yuta Katoa, Tadaaki Arimuraa, Takashi Kuwanoa, Makoto Sugiharaa, Shin-ichiro Miuraa, b
aDepartment of Cardiology, Fukuoka University School of Medicine, Fukuoka, Japan
bCorresponding Author: Shin-ichiro Miura, Department of Cardiology, Fukuoka University School of Medicine, Fukuoka 814-0180, Japan
Manuscript submitted April 12, 2025, accepted June 6, 2025, published online June 16, 2025
Short title: ARNI and Heart Failure
doi: https://doi.org/10.14740/cr2074
- Abstract
- Introduction
- Materials and Methods
- Results
- Changes in body weight (BW), BP, biochemical parameters, and cardiothoracic ratio between baseline and 6 months after ARNI treatment
- Discussion
- References
| Abstract | ▴Top |
Background: This study evaluated the cardioprotective effects of angiotensin receptor-neprilysin inhibitor (ARNI) therapy in patients with heart failure (HF), focusing on blood pressure (BP) and cardiac or renal function.
Methods: A total of 46 patients who started ARNI therapy between December 2020 and March 2023 were included. Blood tests, echocardiography, and assessments of BP and cardiac function including N-terminal pro-brain natriuretic peptide (NT-proBNP) in blood were performed before and 6 months after they started ARNI therapy. The patients were divided into two groups: heart failure with reduced left ventricular (LV) ejection fraction (HFrEF) and non-HFrEF.
Results: Before treatment, the mean NT-proBNP level was 550 pg/mL, LVEF was 45%, and the estimated glomerular filtration rate (eGFR) was 52.7 mL/min/1.73 m2 in all patients. After 6 months of ARNI therapy, NT-proBNP levels significantly decreased to 462 pg/mL (P < 0.01), LVEF improved to 52% (P < 0.01), and BP showed a slight reduction, particularly in patients with high baseline BP. eGFR remained stable (P = 0.53). The results showed that ARNI treatment led to a reduction in NT-proBNP and improvements in cardiac function, with more pronounced effects in patients with HFrEF. BP changes correlated with baseline levels, stabilizing at around 125/70 mm Hg, and there were no significant differences in changes in renal function between HFrEF and non-HFrEF patients.
Conclusions: ARNI therapy significantly reduced NT-proBNP levels and improved cardiac function, with mild antihypertensive effects and no major impact on renal function. These results highlight the importance of predicting the degree of BP reduction by BP at baseline before starting ARNI in HF patients.
Keywords: N-terminal pro-brain natriuretic peptide; Angiotensin receptor-neprilysin inhibitor; Heart failure with reduced left ventricular ejection fraction; Blood pressure
| Introduction | ▴Top |
It is widely recognized that the activation of neurohumoral factors plays a crucial role in the pathogenesis of heart failure (HF). The renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system (SNS) become excessively activated [1, 2], leading to increased secretion of atrial and brain natriuretic peptides (ANP and BNP) from cardiomyocytes, which act to suppress the pathological activation of RAAS and SNS [3]. Angiotensin receptor-neprilysin inhibitor (ARNI) combines the angiotensin receptor blocker (ARB) valsartan with the neprilysin inhibitor sacubitril (sacubitril/valsartan). ARNI not only suppresses RAAS activation but also enhances the compensatory effects of natriuretic peptides (NP) [4-6].
The PARADIGM-HF trial, which targeted HF patients with reduced ejection fraction (HFrEF), demonstrated that ARNI offered superior survival benefits compared to enalapril [7]. In the PARAGON-HF trial, which focused on HF patients with preserved ejection fraction (HFpEF), there was only a slight statistical difference in the composite primary endpoint of HF and cardiovascular death compared to the valsartan group. However, a subgroup analysis revealed that ARNI significantly reduced events in female patients and those with left ventricular ejection fraction (LVEF) ≤ 57% [8]. In the PARALLEL-HF trial, a phase III study conducted in Japanese HFrEF patients, NT-proBNP was significantly reduced, confirming the usefulness of ARNI in Japan [9]. However, in real-world clinical practice, when ARNI is used in HFrEF and HFpEF patients with reduced cardiac function, clinicians often encounter difficulties in transitioning from angiotensin-converting enzyme inhibitors (ACEis) or ARBs and achieving adequate dose-escalation due to low blood pressure (BP) and a high prevalence of chronic kidney disease (CKD).
Therefore, this study evaluated the cardio-renal protective effects of ARNI and its impact on BP and cardiac and renal function in HF patients who had begun to receive ARNI therapy through routine blood tests and cardiac function assessments in daily clinical practice.
| Materials and Methods | ▴Top |
Subjects
This was a prospective observational study. The subjects consisted of 51 HF patients aged 20 years or older who were hospitalized or received outpatient care at Fukuoka University Hospital between December 2020 and March 2023 and newly began treatment with ARNI. Exclusion criteria were as follows: already receiving ARNI therapy, unsuitable for ARNI treatment, and severe hepatic dysfunction (Child-Pugh class C). The diagnosis of HF was made by the attending cardiologists in our University Hospital based on JCS guidelines [10]. Three patients discontinued ARNI therapy because of adverse effects (dizziness, palpitation, and hypotension). Additionally, two patients who were receiving off-label doses were excluded. Finally, we evaluated 46 patients with respect to various parameters before and after ARNI treatment (Fig. 1). We divided the patients into HFrEF (left ventricular ejection fraction (LVEF) < 40%, N = 17) and non-HFrEF (LVEF ≥ 40%, N = 29) groups by transthoracic echocardiogram-derived LVEF at baseline. Non-HFrEF includes HF with both mildly-reduced EF and HFpEF.
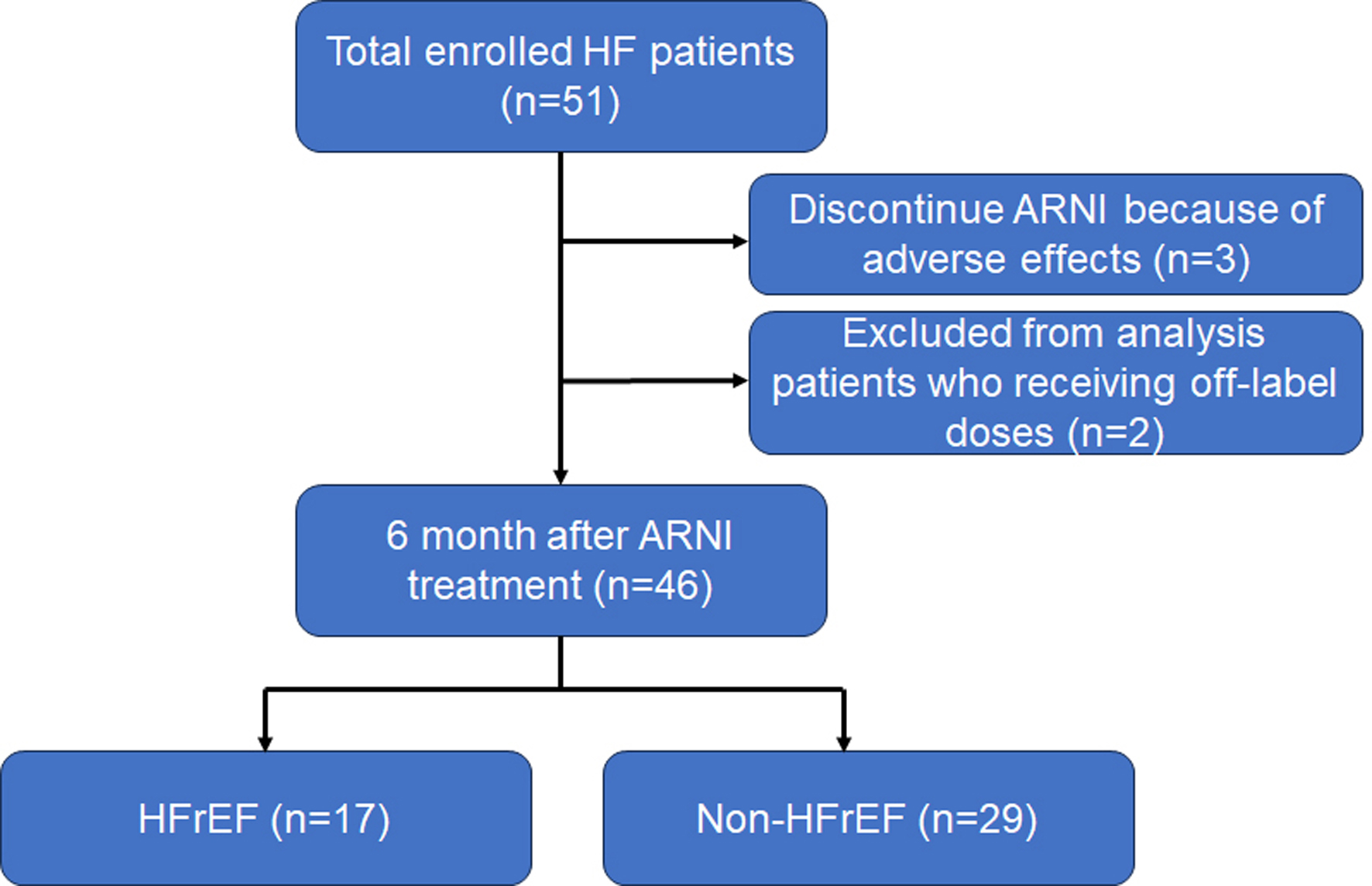 Click for large image | Figure 1. Consort diagram for the present analysis. ARNI: angiotensin receptor-neprilysin inhibitor; HF: heart failure; HFrEF: heart failure with reduced ejection fraction. |
All data were analyzed using the database of Fukuoka University Hospital. The study was performed according to the Declaration of Helsinki regarding investigations in humans and approved by the ethics committee of Fukuoka University.
Primary and secondary endpoints
The primary endpoint was changes in NT-proBNP levels in blood, and secondary endpoints included changes in cardiac or renal function, BP, and the occurrence of major adverse cardiovascular events (MACEs: hospitalization for HF, cardiovascular events, death from cardiovascular causes and new onset of arrhythmia). Cardiovascular events include myocardial infarction (MI), angina pectoris, and unscheduled hospitalization due to cardiovascular diseases.
Assessment of patient characteristics and various parameters
BP, biochemical data (including blood levels of NT-proBNP and estimated glomerular filtration rate (eGFR)), and echocardiography were evaluated before the initiation of ARNI and at 6 months post-initiation. Echocardiographic parameters included LVEF, left ventricular end-diastolic dimension (LVDd), left ventricular end-systolic dimension (LVDs), cardiac output (CO), left ventricular mass index (LVMI), the ratio of early to late diastolic transmitral flow velocity (E/A), the ratio of early transmitral doppler velocity/early diastolic annular velocity (E/e'), left atrial volume index (LAVI), and tricuspid regurgitation pressure gradient (TRPG). CO was estimated from Doppler echocardiographic measurement of left ventricular outflow.
Statistical analysis
Statistical tests were performed using GraphPad Prism 9.5.1 (528) (GraphPad Software, LLC). Values are expressed as the mean ± standard deviation if the variable was normally distributed, and as the median (interquartile range) otherwise. The Shapiro-Wilk test was used to assess whether or not data were normally distributed. Groups were compared using Student’s t-test or the Wilcoxon test for continuous values, and the Chi-square test for trends was used for categorical data. A subgroup analysis was performed to evaluate changes in BP and cardiac or renal function based on the patient background, and correlation was examined using Pearson’s correlation coefficient as appropriate. A two-tailed P < 0.05 was considered statistically significant.
| Results | ▴Top |
Patient characteristics at baseline
Table 1 shows the patient backgrounds in all patients, and in the HFrEF and non-HFrEF groups. The average age was 67 ± 13 years, and 67% of the patients were male. Before treatment, BP was 131 ± 25/78 ± 16 mm Hg, eGFR was 52.7 ± 20 mL/min/1.73 m2, and NT-proBNP was 550 (173 - 1,406) pg/mL. In the HFrEF group, subjective symptoms were more pronounced, NT-proBNP levels were higher, and more patients were initiated during hospitalization for acute HF. Furthermore, 47.1% had ischemic heart disease (IHD), more prior HF hospitalization, and loop diuretic and tolvaptan were more commonly used. Regarding other HF medications, no significant differences in initiation rates were observed between the HFrEF and non-HFrEF groups. There was no resent MI in IHD, only one patient in the HFrEF group received revascularization at the same time, and others were old MI.
 Click to view | Table 1. Characteristics of the Patients at Baseline (N = 46) |
Assessment of primary and secondary endpoints
NT-proBNP significantly decreased to 462 (143 - 1,056) pg/mL after ARNI treatment (P < 0.01) in all patients (Table 2). Particularly in the HFrEF group, NT-proBNP significantly decreased from 1,583 (653 - 4,059) to 678 (386 - 2,388) pg/mL (P < 0.01). On the other hand, in the non-HFrEF group, NT-proBNP decreased from 365 (120 - 714) to 199 (118 - 806) pg/mL, but this change was not significant (P = 0.28). Three of the 46 total patients had MACE within 6 months after ARNI treatment, all of whom were in the HFrEF group (one with cardiovascular events and two with new onset of arrhythmia) (HFrEF group vs. non-HFrEF, P = 0.027). Case 1: Fourteen days after ARNI therapy, the patient experienced an episode of angina pectoris attributed to coronary artery spasm. Treatment with diltiazem at a dose of 100 mg/day was initiated, and no recurrence of anginal symptoms was observed thereafter. Case 2: Two months after the commencement of ARNI therapy, the patient developed sustained ventricular tachycardia requiring multiple shocks from an implantable cardioverter-defibrillator (ICD). The dosage of amiodarone was increased from 50 to 100 mg/day, after which no further ICD shocks were reported. Case 3: On day 7 following ARNI therapy, ICD interrogation revealed an episode of ventricular fibrillation. Amiodarone therapy was initiated at 200 mg/day and subsequently reduced to 100 mg/day after 1 month. No recurrence of ventricular arrhythmias was observed thereafter.
| Changes in body weight (BW), BP, biochemical parameters, and cardiothoracic ratio between baseline and 6 months after ARNI treatment | ▴Top |
At 6 months after the initiation of administration, mean BP was 126 ± 23 (P = 0.10)/72 ± 15 mm Hg (P = 0.03), and eGFR was 51.8 ± 21.7 mL/min/1.73 m2 in all patients (P = 0.53) (Table 2). The reduction in BP was modest and did not significantly affect renal function; similar findings were seen in the HFrEF and non-HFrEF groups.
 Click to view | Table 2. Change in BW, BP, Biochemical Parameters, and Cardiothoracic Ratio Between Baseline and 6 Months After ARNI Treatment |
Changes in systolic BP (SBP) and diastolic BP (DBP) at baseline and after 6 months in each patient
Figure 2 shows SBP and DBP values at baseline and after 6 months in each patient. One patient in both HFrEF and non-HFrEF groups with very high baseline SBP and DBP had tremendous decreases in SBP and DBP, whereas changes in other patients’ BP were very minimal.
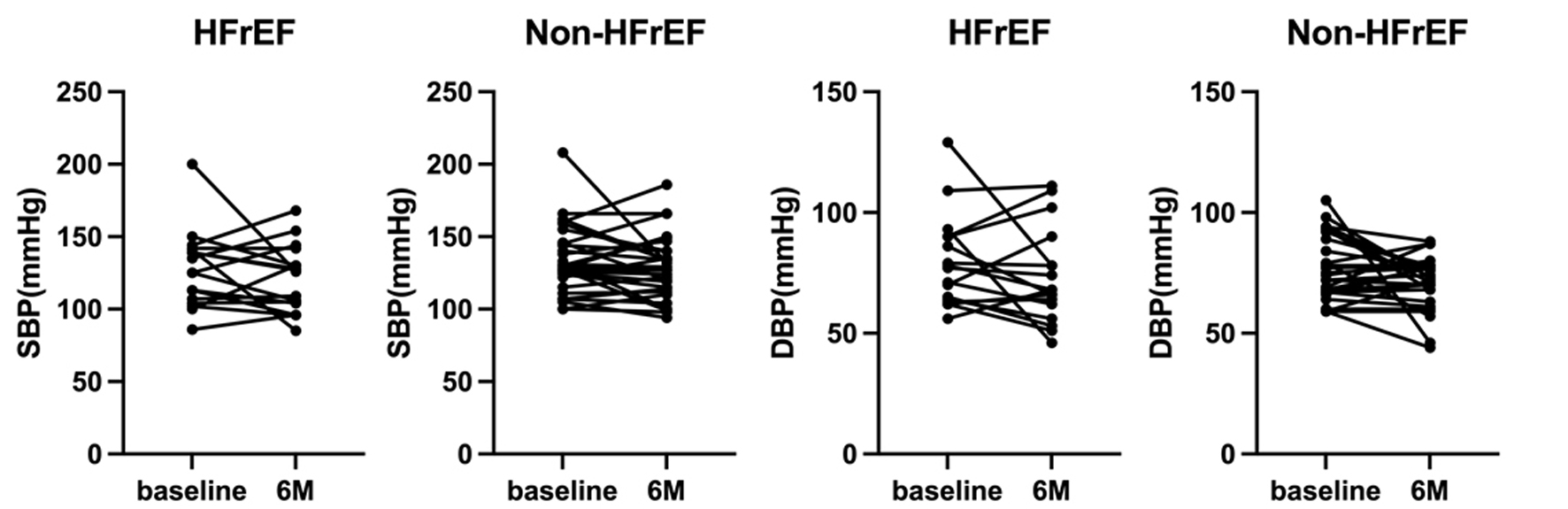 Click for large image | Figure 2. Changes in SBP and DBP at baseline and after 6 months (M) in each patient. DBP: diastolic blood pressure; HFrEF: heart failure with reduced ejection fraction; SBP: systolic blood pressure. |
Associations between changes in SBP or DBP from baseline to 6 months (ΔSBP or ΔDBP) and SBP or DBP at baseline
As shown in Figure 3, ΔSBP and ΔDBP were significantly negatively associated with SBP (r = 0.55, P < 0.01) and DBP (r = 0.60, P < 0.01) at baseline, respectively, although changes in SBP and DBP were not consistent (Fig. 2).
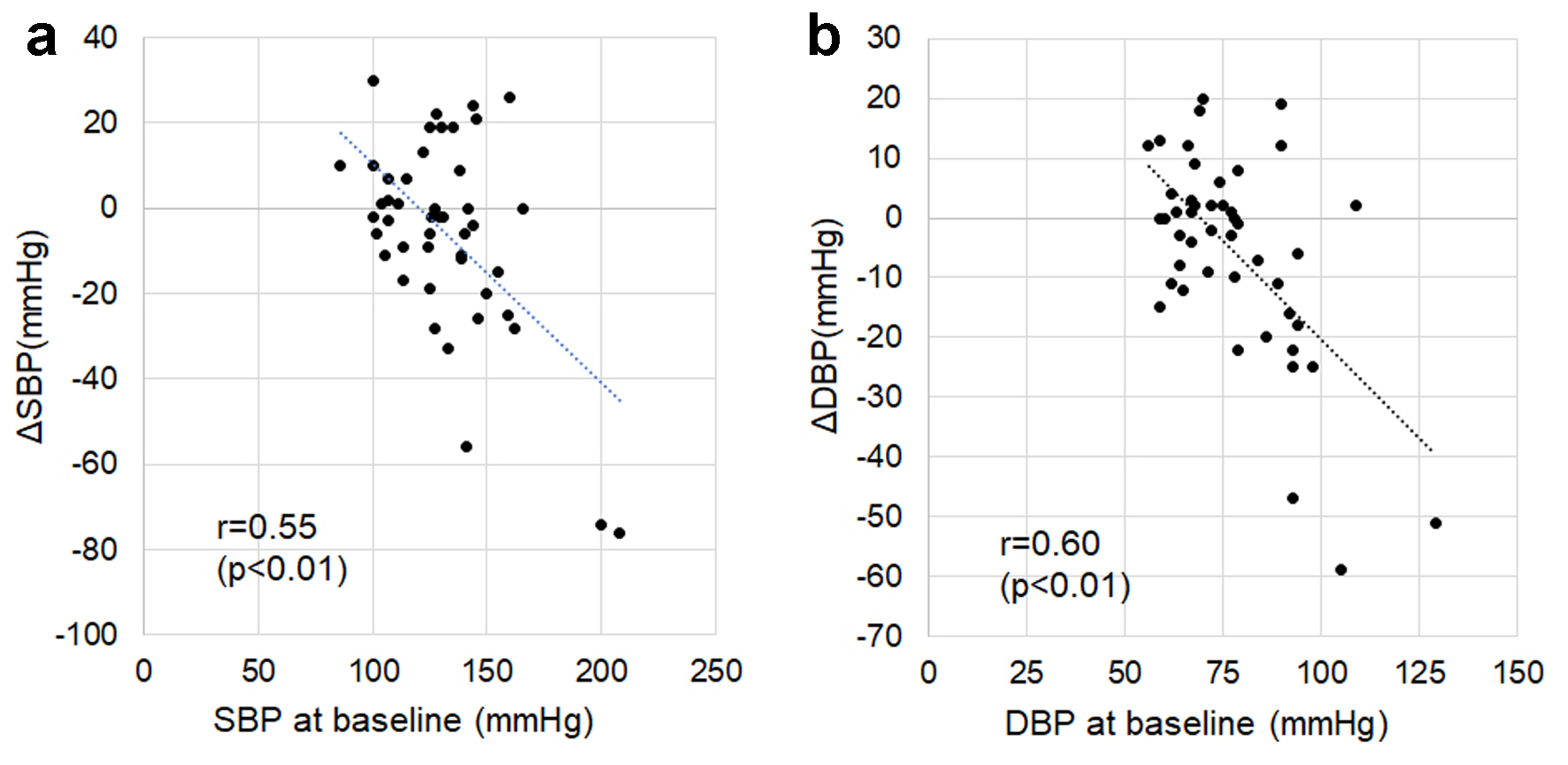 Click for large image | Figure 3. Associations between changes in SBP or DBP from baseline to 6 months after ARNI treatment (ΔSBP or ΔDBP) and SBP (a) or DBP (b) at baseline. ARNI: angiotensin receptor-neprilysin inhibitor; DBP: diastolic blood pressure; SBP: systolic blood pressure. |
Changes in echocardiographic parameters between baseline and 6 months after ARNI treatment
LVEF improved to 52±15% after ARNI treatment in all patients (P < 0.01). LVDd decreased from 53.2 (46.5 - 56.3) to 49.3 (44.8 - 54.1) mm (P < 0.01), and LVDs decreased from 41.8 (33.4 - 48.6) to 36.4 (29.4 - 42.3) mm (P < 0.01). Particularly in HFrEF patients, after the initiation of ARNI, LVEF significantly improved (P < 0.01), along with cardiac enlargement (P < 0.01) (Table 3).
 Click to view | Table 3. Change in Echocardiographic Parameter Between Baseline and 6 Months After ARNI Treatment |
Associations between ΔNT-proBNP and NT-proBNP or cardiac parameters at baseline
A strong negative correlation was observed between ΔNT-proBNP and NT-proBNP at baseline (r = -0.92, P < 0.01) in Figure 4. We also evaluated the relationship with echocardiographic parameters related to cardiac function (LVEF and CO), and cardiac dilation (LVDd and LVDs). There were no correlations between ΔNT-proBNP and LVEF, CO, LVDd, or LVDs at baseline. There was also no correlation between ΔNT-proBNP and LVMI at baseline (Fig. 4).
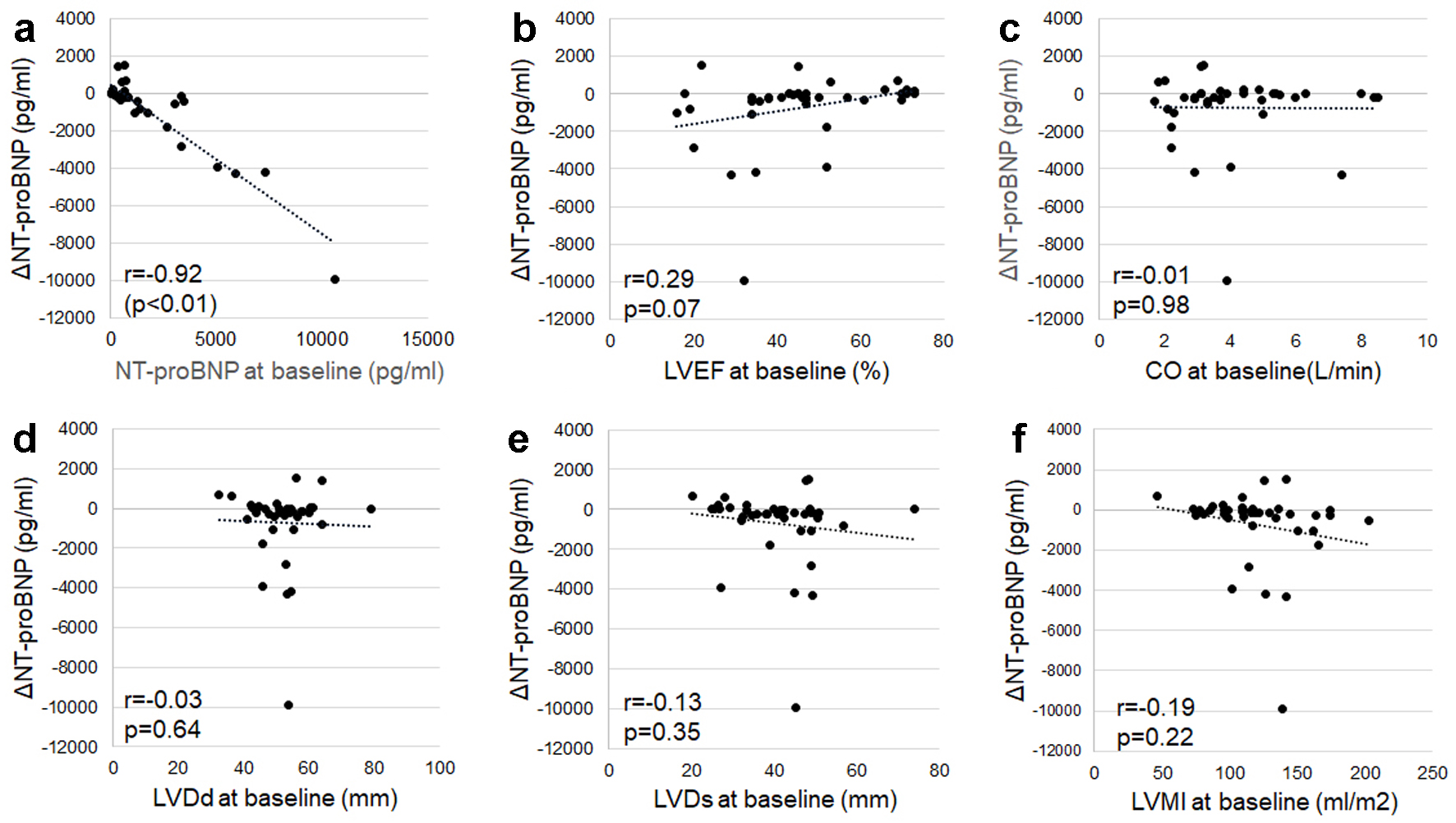 Click for large image | Figure 4. Associations between changes in NT-proBNP from baseline to 6 months after ARNI treatment (ΔNT-proBNP) and NT-proBNP (a) or echocardiographic parameters (b-f) at baseline. Echocardiographic parameters related to cardiac function (LVEF (b), CO (c)), and cardiac dilation (LVDd (d), LVDs (e), and LVMI (f)) at baseline were used. ARNI: angiotensin receptor-neprilysin inhibitor; CO: cardiac output; LVDd: left ventricular end-diastolic dimension; LVDs: left ventricular end-systolic dimension; LVEF: left ventricular ejection fraction; LVMI: left ventricular mass index; NT-proBNP: N-terminal pro-brain natriuretic peptide. |
Associations between ΔNT-proBNP and indicators of left ventricular diastolic dysfunction and high left atrial pressure at baseline
Moderate to weak negative associations were observed between ΔNT-proBNP and E/A (r = -0.49, P < 0.01), E/e' (r = -0.49, P < 0.01), LAVI (r = -0.36, P = 0.03), and TRPG (r = -0.35, P = 0.05) at baseline (Fig. 5).
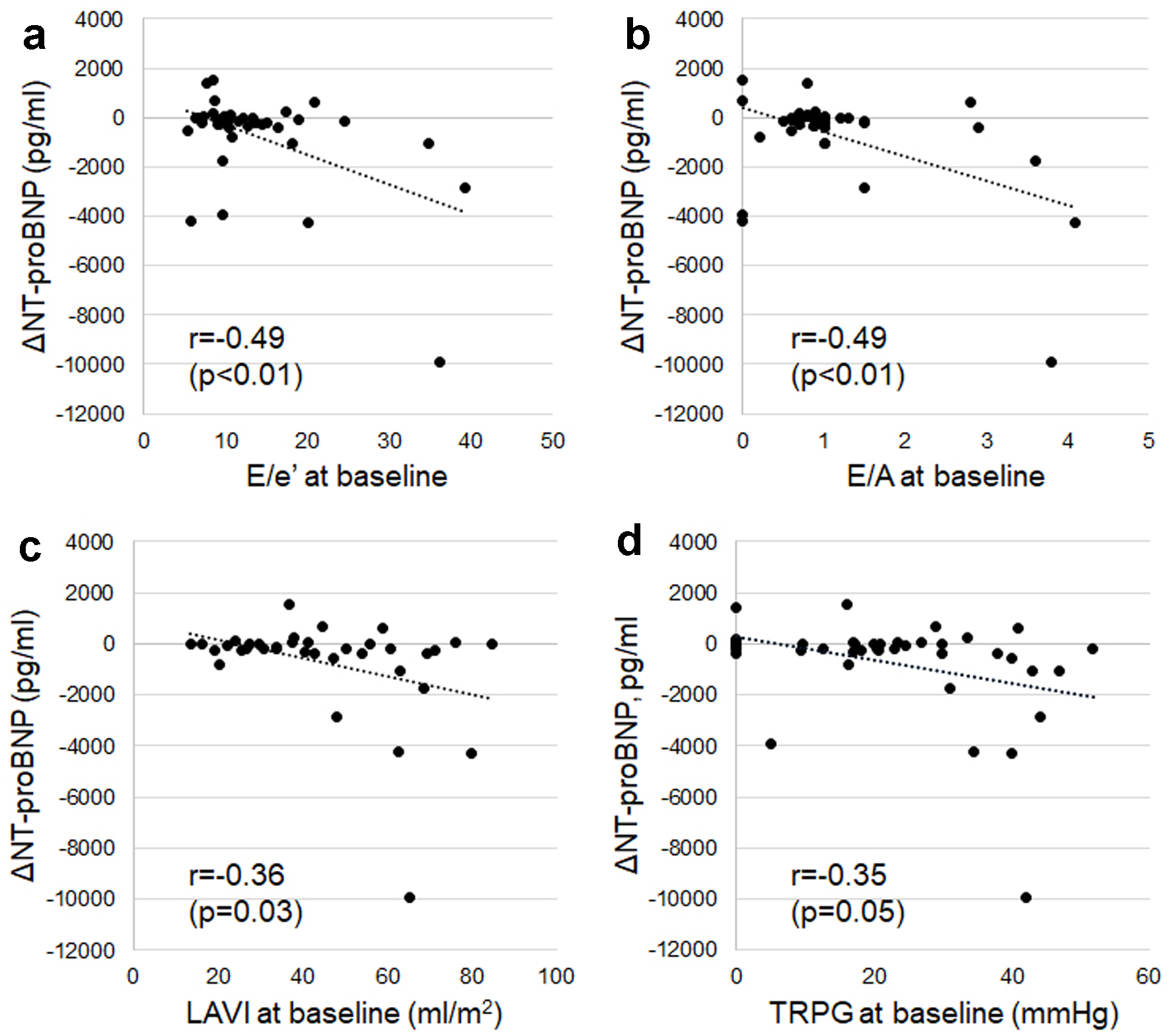 Click for large image | Figure 5. Associations between changes in NT-proBNP from baseline to 6 months after ARNI treatment (ΔNT-proBNP) and indicators of left ventricular diastolic dysfunction and high left atrial pressure (E/e' (a), E/A (b), LAVI (c), and TRPG (d)) at baseline. E/A: ratio of early to late diastolic transmitral flow velocity; E/e': ratio of early transmitral Doppler velocity to early diastolic annular velocity; LAVI: left atrial volume index; NT-proBNP: N-terminal pro-brain natriuretic peptide; TRPG: tricuspid regurgitation pressure gradient. |
Changes in NT-proBNP and LVEF at baseline and after 6 months in each patient
Figure 6 shows NT-proBNP levels and LVEF at baseline and after 6 months in each patient. There was one “super responder” whose NT-proBNP decreased by 10,000 pg/mL. This HFrEF patient had poor renal function and a very high apparent NT-proBNP value at baseline, which tremendously improved after 6 months.
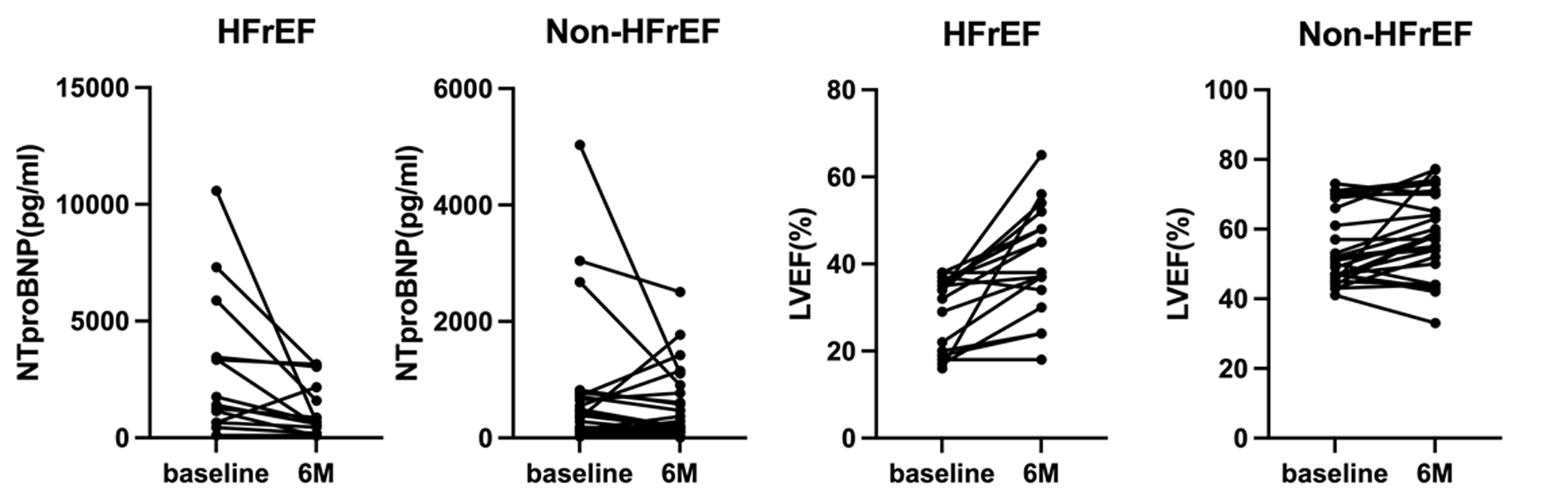 Click for large image | Figure 6. Changes in NT-proBNP and LVEF at baseline and after 6 months (M) in each patient. LVEF: left ventricular ejection fraction; NT-proBNP: N-terminal pro-brain natriuretic peptide. |
Associations between ΔNT-proBNP levels or NT-proBNP levels at baseline and ΔSBP/ΔDBP or SBP/DBP at baseline
There were no correlations between the ΔNT-proBNP levels or NT-proBNP levels at baseline and ΔSBP/ΔDBP or SBP/DBP at baseline (Fig. 7).
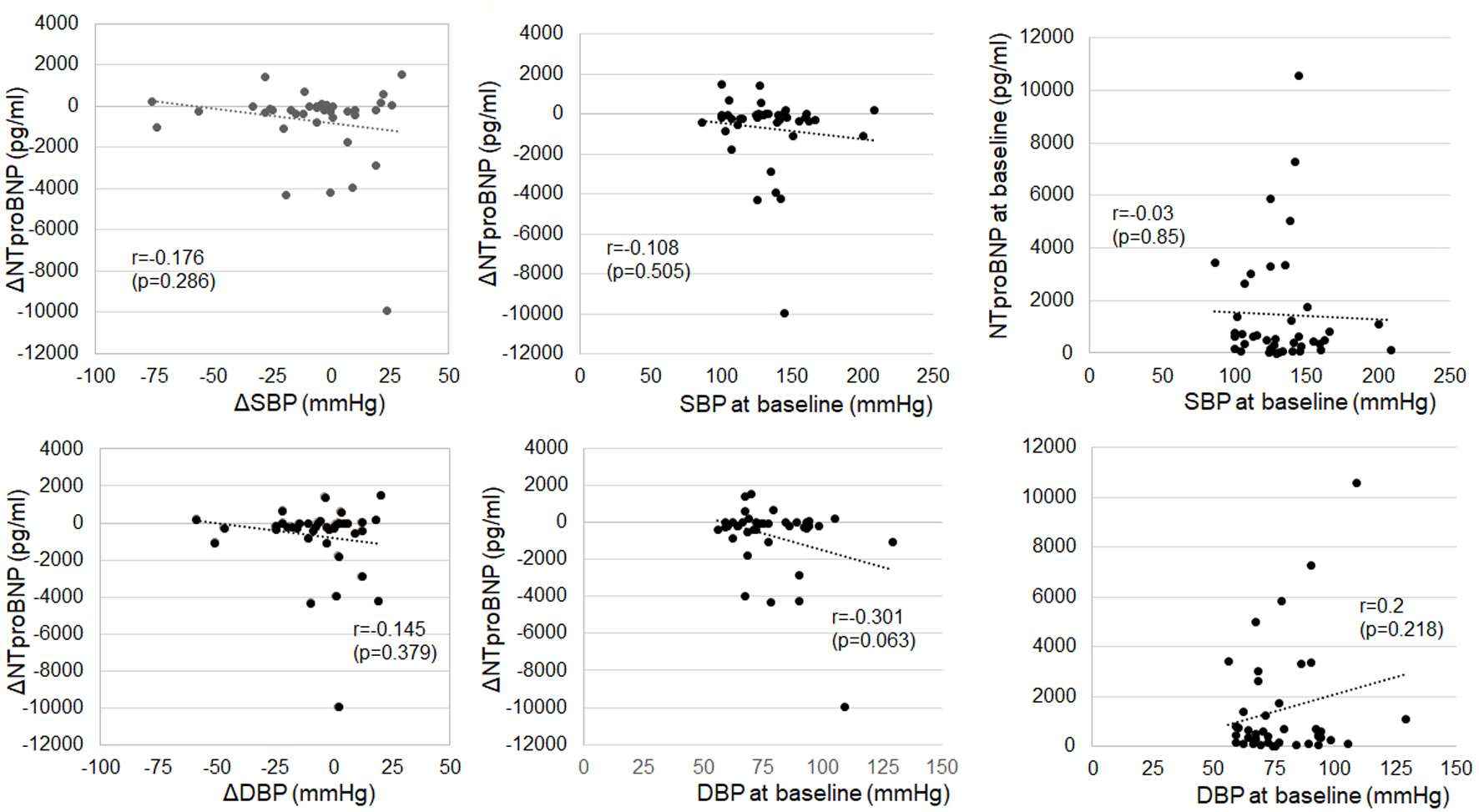 Click for large image | Figure 7. Associations between changes in NT-proBNP from baseline to 6 months after ARNI treatment (ΔNT-proBNP) or NT-proBNP levels at baseline and ΔSBP/ΔDBP or SBP/DBP at baseline. ARNI: angiotensin receptor-neprilysin inhibitor; DBP: diastolic blood pressure; NT-proBNP: N-terminal pro-brain natriuretic peptide; SBP: systolic blood pressure. |
Associations between doses of ARNI and changes in ΔNT-pro-BNP or ΔLVEF
Twenty patients received ARNI 100 mg/day (nine and 11 patients were HFrEF and non-HFrEF, respectively), 20 patients received ARNI 200 mg/day (six and 14 patients were HFrEF and non-HFrEF, respectively), and six patients received 400 mg/day (two and four patients were HFrEF and non-HFrEF, respectively). The doses of ARNI are not associated with changes in LVEF and NT-proBNP (Fig. 8).
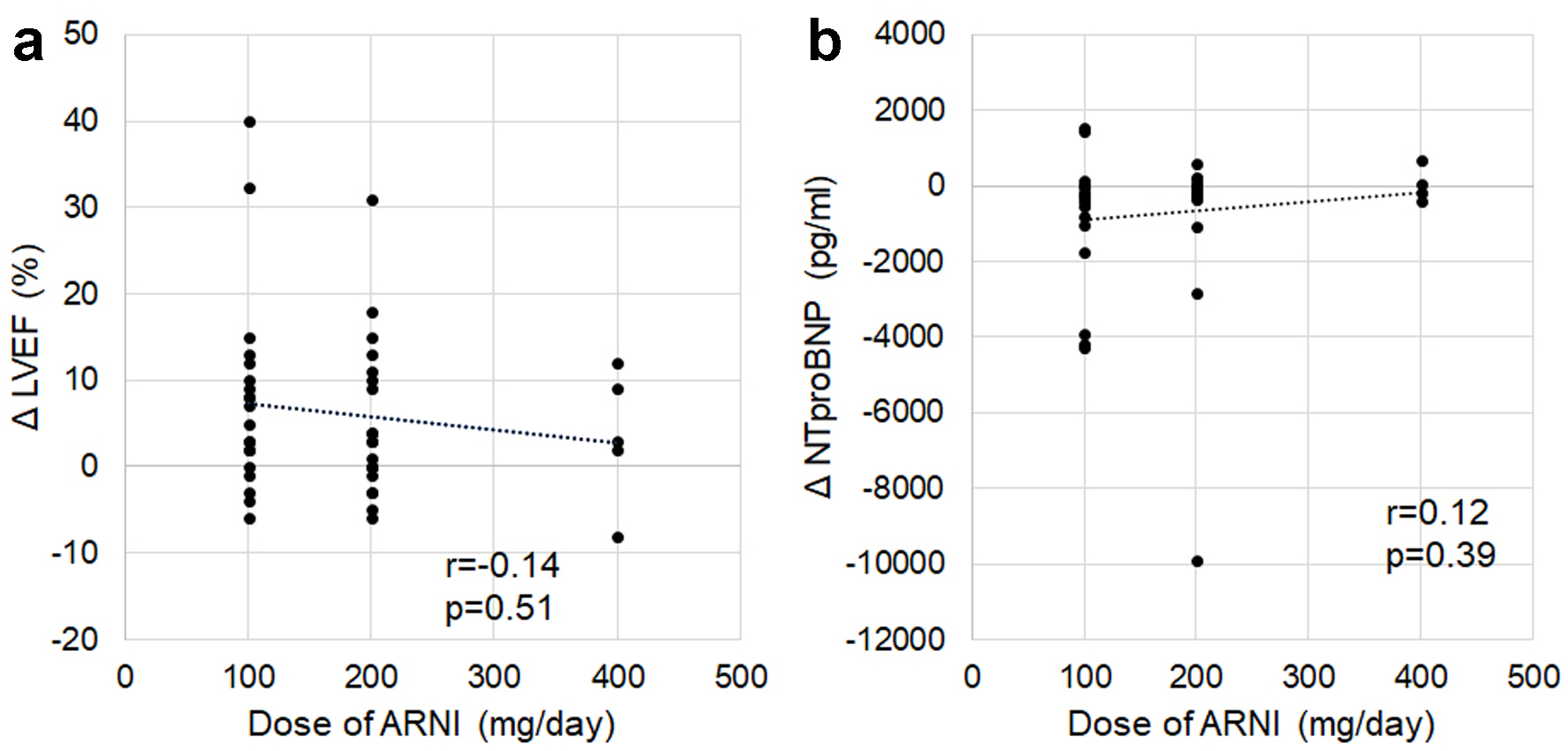 Click for large image | Figure 8. Associations between doses of ARNI and changes in ΔLVEF (a) and ΔNT-proBNP (b). ARNI: angiotensin receptor-neprilysin inhibitor; LVEF: left ventricular ejection fraction; NT-proBNP: N-terminal pro-brain natriuretic peptide. |
| Discussion | ▴Top |
ARNI treatment in patients with HF significantly reduced NT-proBNP, particularly in patients with HFrEF, and improvements in cardiac function and cardiac enlargement were observed. The reduction in BP was modest and was not associated with significant changes in renal function, with no significant differences noted between the HFrEF and non-HFrEF groups. This study found that while the primary endpoints for HFrEF improved, the primary endpoints for HFpEF did not, and similar results were observed in real-world settings.
The significant reduction in NT-proBNP observed in patients with HFrEF warrants further investigation. To explore this, we investigated the association between changes in NT-proBNP levels (ΔNT-proBNP) and baseline NT-proBNP concentrations, as well as baseline echocardiographic parameters. We included echocardiographic indices related to cardiac function and chamber dilation, which are commonly associated with HF. A strong negative correlation was observed between ΔNT-proBNP and NT-proBNP at baseline. In contrast, no correlation was found between ΔNT-proBNP and LVEF, CO, LVDd, LVDs, or LVMI at baseline. A moderate to weak negative correlation was observed between ΔNT-proBNP and E/A, E/e', LAVI or TRPG at baseline, which are indicators of HF condition and diastolic dysfunction, respectively. The PIONEER-HF trial has demonstrated the efficacy of ARNI in acute HF. In patients with acute decompensated HF (HFrEF patients), administration of ARNI before discharge, after hemodynamic stabilization, resulted in a significant reduction in NT-proBNP at 8 weeks post-discharge compared to an enalapril monotherapy group [11]. The TRANSITION trial also examined the introduction of ARNI during hospitalization [12]. In the acute phase of HF, elevated left atrial pressure and fluid overload are common; thus, the diuretic effect of neprilysin inhibition may offer therapeutic benefits. These findings suggest that ARNI may exert greater efficacy in patients with elevated left atrial pressure.
In this study, no group exhibited a decline in LVEF after treatment; instead, LVEF either remained unchanged or improved. In the HFrEF group, 6 months after initiating ARNI, LVMI decreased by 25 mL/m2 and LVEF increased by 12%. Our previous basic studies demonstrated that sacubitril in ARNI exerts a diuretic effect, while valsartan contributes to inhibiting cardiac hypertrophy [13, 14]. Regarding the anti-hypertrophic effects observed in this study, posterior wall thickness of left ventricle was reduced, but the difference was not statistically significant (data not shown). Given the small sample size, the results of our study need further validation and evaluation in a larger prospective study.
As noted above, after ARNI treatment, NT-proBNP significantly decreased and cardiac function and cardiac enlargement improved in the HFrEF group. On the other hand, a statistically significant number of MACEs were seen in the HFrEF group, and the suppression of events was inadequate. A possible explanation for this result is that NT-proBNP levels at baseline in the HFrEF group were significantly higher than those in the non-HFrEF group, and the control of HF in the HFrEF group at the start of the study may have been worse than that in the non-HFrEF group. We need to reconsider the timing of ARNI initiation and how to increase the dose.
In this study, ΔBP after ARNI treatment in HF patients correlated with BP at baseline, and mean BP values converged to 126/72 mm Hg following ARNI treatment. On the other hand, previous studies have shown that baseline New York Heart Association (NYHA) functional class III or IV, SBP < 100 mm Hg, and eGFR < 30 mL/min/1.73 m2 were significantly associated with both the occurrence of adverse events and the discontinuation of ARNI. Furthermore, patients who experienced adverse events had a higher risk of cardiovascular death or HF-related hospitalization compared to those without adverse events [15]. According to this study, in HF patients whose baseline BP is relatively well maintained before treatment, it is advisable to aim for dose escalation up to the target dose. However, caution is required regarding symptomatic hypotension, especially in patients with lower BP at baseline. Moreover, in this study, all three patients in whom ARNI administration was discontinued had a poor prognosis (one patient had worsening of HF with cardiac resynchronization therapy defibrillator implantation, one had palpitation and died of cancer after 6 months, and one had infective endocarditis and died of sepsis after 8 months). A sub-group analysis in the PARADIGM-HF trial also demonstrated that the dose-reduction group had higher risks of cardiovascular events and HF-related hospitalization [16]. If ARNI dose reduction or discontinuation becomes necessary, the prognosis may be poor, and closer attention should be paid to the subsequent clinical course.
Study limitations
Our study has several limitations that should be considered. First, since there is no control group in this study, it is not possible to consider patients with or without ARNI administration. Second, our study was performed at a single institution with a limited number of study patients, and there was a potential selection bias. Third, the results of our study may have been affected by the small sample size. The sample size should have been designed by calculation. In future work, the results of our study need to consider regression modeling in a larger prospective study. Finally, we did not consider the impact of changes in other HF medications. During the study period, changes in other HF medications were observed in 13 cases (88.2%) of HFrEF and 12 cases (41.4%) of non-HFrEF. At baseline, 13 patients (28.3%) were receiving sodium-glucose cotransporter-2 inhibitor (SGLT2i) and 24 (52.2%) were receiving mineralocorticoid receptor antagonist (MRA). By 6 months, SGLT2i and MRA were introduced in five cases each. Conversely, one patient had discontinued MRA by 6 months. There were no significant differences in the changes of eco parameters (ΔLVEF, ΔLVDd, and ΔLVDs) between the two groups with and without SGLT2i administration at baseline. These were similar between the two groups with and without MRA administration at baseline. In the MRA introduction group, there was a significant difference in ΔLVEF. Otherwise, there were no significant differences in echo parameters (ΔLVEF, ΔLVDd, and ΔLVD for SGLT2i and ΔLVDd and ΔLVDs for MRA). The details of these data are not shown in this report. The influence of other medications on these results requires further investigation.
Conclusion
ARNI contributed to the reduction of NT-proBNP and the improvement of cardiac function, and its antihypertensive effects and impact on renal function were minimal. When introducing ARNI therapy in HF patients, it is crucial to assess baseline BP, NT-proBNP levels, and signs of elevated left atrial pressure. Findings from previous studies in addition to the present study suggest that when dose reduction or discontinuation becomes necessary, the prognosis may worsen, requiring closer monitoring of the subsequent clinical course.
Acknowledgments
The authors thank all the investigators involved in this study.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no financial conflict of interest.
Informed Consent
Written informed consent was obtained from all patients before starting the study.
Author Contributions
Conceptualization: HM, Y. Shiga, and SM; methodology: HM and Y. Shiga; validation: HM and Y. Shiga; formal analysis: HM and Y. Shiga; investigation: HM, Y. Shiga, YK, TA, and Y. Suematsu; data curation: HM and Y. Shiga; writing - original draft preparation: HM and Y. Shiga; writing - review and editing: SM; visualization: TK and MS; supervision: SM.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Dzau VJ. Tissue renin-angiotensin system in myocardial hypertrophy and failure. Arch Intern Med. 1993;153(8):937-942.
pubmed - Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341(8):577-585.
doi pubmed - Mancini GB, Howlett JG, Borer J, Liu PP, Mehra MR, Pfeffer M, Swedberg K, et al. Pharmacologic options for the management of systolic heart failure: examining underlying mechanisms. Can J Cardiol. 2015;31(10):1282-1292.
doi pubmed - Bayes-Genis A, Barallat J, Richards AM. A test in context: neprilysin: function, inhibition, and biomarker. J Am Coll Cardiol. 2016;68(6):639-653.
doi pubmed - Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010;50(4):401-414.
doi pubmed - Volpe M, Rubattu S, Battistoni A. ARNi: a novel approach to counteract cardiovascular diseases. Int J Mol Sci. 2019;20(9):2092.
doi pubmed - McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004.
doi pubmed - Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609-1620.
doi pubmed - Tsutsui H, Momomura S, Saito Y, Ito H, Yamamoto K, Ohishi T, Okino N, et al. Efficacy and safety of sacubitril/valsartan (LCZ696) in Japanese patients with chronic heart failure and reduced ejection fraction: Rationale for and design of the randomized, double-blind PARALLEL-HF study. J Cardiol. 2017;70(3):225-231.
doi pubmed - Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, Kitakaze M, et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure - digest version. Circ J. 2019;83(10):2084-2184.
doi pubmed - Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539-548.
doi pubmed - Wachter R, Senni M, Belohlavek J, Straburzynska-Migaj E, Witte KK, Kobalava Z, Fonseca C, et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019;21(8):998-1007.
doi pubmed - Suematsu Y, Miura S, Goto M, Matsuo Y, Arimura T, Kuwano T, Imaizumi S, et al. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur J Heart Fail. 2016;18(4):386-393.
doi pubmed - Tashiro K, Kuwano T, Ideishi A, Morita H, Idemoto Y, Goto M, Suematsu Y, et al. Sacubitril/valsartan inhibits cardiomyocyte hypertrophy in angiotensin ii-induced hypertensive mice independent of a blood pressure-lowering effect. Cardiol Res. 2020;11(6):376-385.
doi pubmed - Matsumoto S, McMurray JJV, Nasu T, Ishii S, Kagiyama N, Kida K, Fujimoto W, et al. Relevant adverse events and drug discontinuation of sacubitril/valsartan in a real-world Japanese cohort: REVIEW-HF registry. J Cardiol. 2024;84(2):133-140.
doi pubmed - Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, Teerlink JR, et al. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18(10):1228-1234.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.