| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Short Communication
Volume 16, Number 5, October 2025, pages 447-452
Evaluation of a Point-of-Care N-Terminal Pro-Brain Natriuretic Peptide Assay for Heart Failure Management
Florian Belika, Meryem Benamoura, Antoine Laffalizea, Thibault Lavalleyea, Louisa Van Bellea, Anne-Catherine Pouleurb, Damien Grusona, c
aDepartment of Clinical Chemistry, Cliniques Universitaires Saint-Luc, UCLouvain, Brussels, Belgium
bDivision of Cardiology, Department of Cardiovascular Diseases, Cliniques Universitaires St. Luc and Pole de Recherche Cardiovasculaire, Institut de Recherche Experimentale et Clinique, Cardiovascular Division, Universite Catholique de Louvain, Ottignies-Louvain-la-Neuve, Belgium
cCorresponding Author: Damien Gruson, Department of Clinical Chemistry, Cliniques Universitaires Saint-Luc, UCLouvain, 1200 Brussels, Belgium
Manuscript submitted July 17, 2025, accepted September 3, 2025, published online October 10, 2025
Short title: NT-proBNP Assay for HF Management
doi: https://doi.org/10.14740/cr2117
| Abstract | ▴Top |
Background: N-terminal pro-brain natriuretic peptide (NT-proBNP) is a key biomarker in heart failure (HF) diagnosis and management. This study aimed to evaluate performances of the LumiraDx® NT-proBNP, a point-of-care testing (POCT) device, focusing on imprecision, method comparison, and clinical practicability.
Methods: The LumiraDx® NT-proBNP test was assessed for imprecision across two reagent lots and compared with a reference laboratory method (Cobas e601) using 81 plasma samples. Method concordance was analyzed using Bland-Altman and Passing-Bablok regression. A user satisfaction survey evaluated its practicality in a clinical setting.
Results: For the first reagent lot, a coefficient of variation (CV) of 2.81% was observed, while for the second reagent lot, the CV was 5.4%. Method comparison revealed strong concordance with the reference method for NT-proBNP values < 1,000 ng/L. However, a significant bias was observed for values > 1,000 ng/L in the first lot, resolved in the second. User satisfaction surveys highlighted ease of use. Additionally, implementing the LumiraDx® NT-proBNP Platform resulted in a significant reduction in turnaround time, with an estimated 49 min saved in result reporting.
Conclusion: The LumiraDx® NT-proBNP POCT device demonstrates strong potential for HF management by combining rapid results, user-friendly operation, and sampling versatility. While biases at higher NT-proBNP levels warrant further standardization, this system represents a practical tool for decentralized HF care.
Keywords: NT-proBNP; Point-of-care testing; Cardiovascular disease; Heart failure; Biomarker
| Introduction | ▴Top |
N-terminal pro-brain natriuretic peptide (NT-proBNP) is a key biomarker in the management of heart failure (HF), a condition characterized by the heart’s inability to pump blood effectively [1]. HF often results from structural or functional abnormalities of the heart, and it manifests through symptoms like shortness of breath, fatigue, and fluid retention. NT-proBNP testing is widely recommended by international guidelines for diagnosing HF and monitoring treatment efficacy [1, 2]. Natriuretic peptide levels are used as an initial diagnostic tool to rule out HF in symptomatic patients, with NT-proBNP levels below 125 ng/L making the diagnosis unlikely. Beyond its diagnostic role, NT-proBNP levels are closely linked to the severity of HF, offering valuable prognostic insights. Natriuretic peptides are also emerging as a tool for the screening of HF in high-risk populations such as diabetes [3]. Additionally, NT-proBNP is essential for monitoring treatment response, particularly with new therapies like angiotensin receptor-neprilysin inhibitors (ARNIs), as its levels remain unaffected by these drugs, ensuring accurate tracking of disease progression [1, 4, 5].
While NT-proBNP testing is commonly conducted using automated immunoassays in central laboratories, point-of-care testing (POCT) has emerged as a valuable alternative. POCT is conducted directly at the patient’s bedside, away from the central laboratory, providing rapid and accurate results. This decentralized approach can significantly reduce turnaround time (TAT), which is especially beneficial in managing HF. By enabling quicker decision-making in both emergency situations and routine follow-ups, POCT allows for more timely treatment adjustments and interventions, ultimately improving patient care. POCT offers also new opportunities for improving the access to the natriuretic peptides and contributing to early diagnosis of HF in primary care settings [6].
This study evaluates the performance of the LumiraDx® Platform for NT-proBNP testing, focusing on imprecision, comparison with a reference laboratory method, and assessment of practicality in a clinical setting.
| Materials and Methods | ▴Top |
The LumiraDx® NT-proBNP test is a rapid microfluidic immunofluorescence assay for quantitative NT-proBNP measurement in whole blood or plasma. This POCT requires only 20 µL of sample to measure NT-proBNP, offering a measurement range from 50 to 9,000 ng/L and accommodating a hematocrit range of 15% to 55%, with the flexibility to use either fingerstick or venous blood samples. Regarding interferences, hemoglobin (up to 216 mg/dL), lipemia (up to 1,320 mg/dL), and bilirubin (up to 40 mg/dL) in samples have shown no significant interferent effect on NT-proBNP results [7]. The assays were performed according to the manufacturer’s instructions: 20 µL of the heparinized plasma sample was dispensed onto the sample application area using a pipette. After 12 min, the result was displayed on the instrument screen.
Imprecision was evaluated through the reproducibility (inter-run coefficient of variation (CV)) of the method. For this purpose, one level of control was tested twice a day over five consecutive days with a first lot of reagents and using the LumiraDx® NT-proBNP control. The process was then repeated with a second lot of reagents, using the Liquid Check Cardiac L2 control from Bio-Rad®.
For the method comparison, a total of 81 heparinized plasma samples, originally requested for NT-proBNP testing, were initially processed using our reference method, the electrochemiluminescence immunoassay (ECLIA) on the Cobas e601 module (Roche Diagnostics, Germany), following the manufacturer’s instructions. These samples were subsequently analyzed using the LumiraDx® Platform system within 24 h. Of these 81 samples, 43 were tested using LumiraDx NT-proBNP first reagent lot, and 38 were tested using the second reagent lot. All plasma samples (n = 81) were collected consecutively between at a single tertiary care facility, from patients referred for NT-proBNP testing in the context of suspected or established HF. Thus, the study population represents a real-world hospital cohort rather than healthy individuals. The concordance between the Cobas e601 and LumiraDx® Platform was evaluated using Bland-Altman plots and Passing-Bablok regression analysis, with statistical computations performed using MedCalc software. The study was approved by the institutional review board of Cliniques Universitaires Saint-Luc, Brussels. All procedures were conducted in accordance with the Declaration of Helsinki. Residual plasma samples originally collected for routine NT-proBNP testing were used, and informed consent was obtained from all patients.
To assess the practicability of the LumiraDx® Platform in a clinical setting, we conducted a survey involving 11 healthcare professionals using a structured questionnaire, based on the Scandinavian evaluation of laboratory equipment for point-of-care testing (SKUP) guidelines. Each of the 11 questions required the participants to assign a rating, satisfactory, intermediate, or unsatisfactory. The goal for user-friendliness was to achieve an overall satisfactory score.
| Results | ▴Top |
For the imprecision study, we obtained a CV of 2.81% for the first reagent lot, which is considered acceptable as it falls below the maximum allowable CV according to Westgard criteria (5%), with a mean quality control (QC) value of 109.3 ng/L. The second reagent lot yielded a mean value of 159.7 ng/L and a CV of 5.40%, slightly above Westgard criteria.
For the first reagent lot, a total of 43 samples were evaluated. However, six samples were excluded from the method comparison due to being outside the measuring range of the LumiraDx® Platform: five samples had NT-proBNP measured < 50 ng/L, and one sample had a level > 9,000 ng/L.
The results of the method comparison findings for the different lots are presented in Figure 1 and Figure 2 and the data from the Passing and Bablok regression analysis and Bland-Altman analysis are summarized in Table 1.
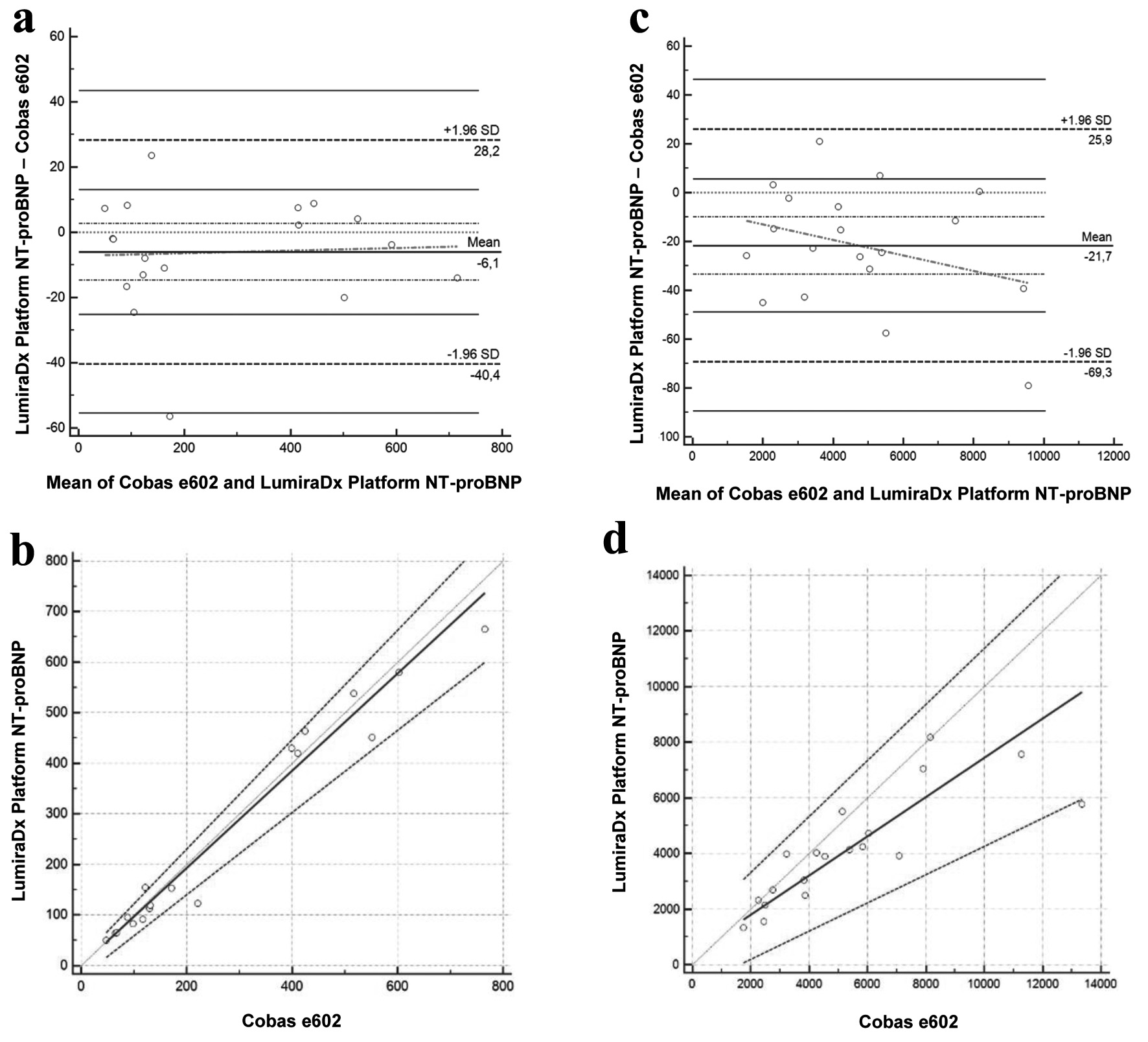 Click for large image | Figure 1. Bland-Altman and Passing-Bablok for the LumiraDx Platform NT-proBNP assay with the first reagent lot. Bland-Altman (a) and Passing-Bablok (b) of values < 1,000 ng/L, for the NT-proBNP LumiraDx Platform assay compared with the gold standard Cobas e602 assay (n = 18). Bland-Altman (c) and Passing-Bablok (d) of values > 1,000 ng/L, for the NT-proBNP LumiraDx Platform assay compared with the gold standard Cobas e602 assay (n = 19). *The units are ng/L and % for Passing-Bablok and Bland-Altman, respectively. NT-proBNP: N-terminal pro-brain natriuretic peptide. |
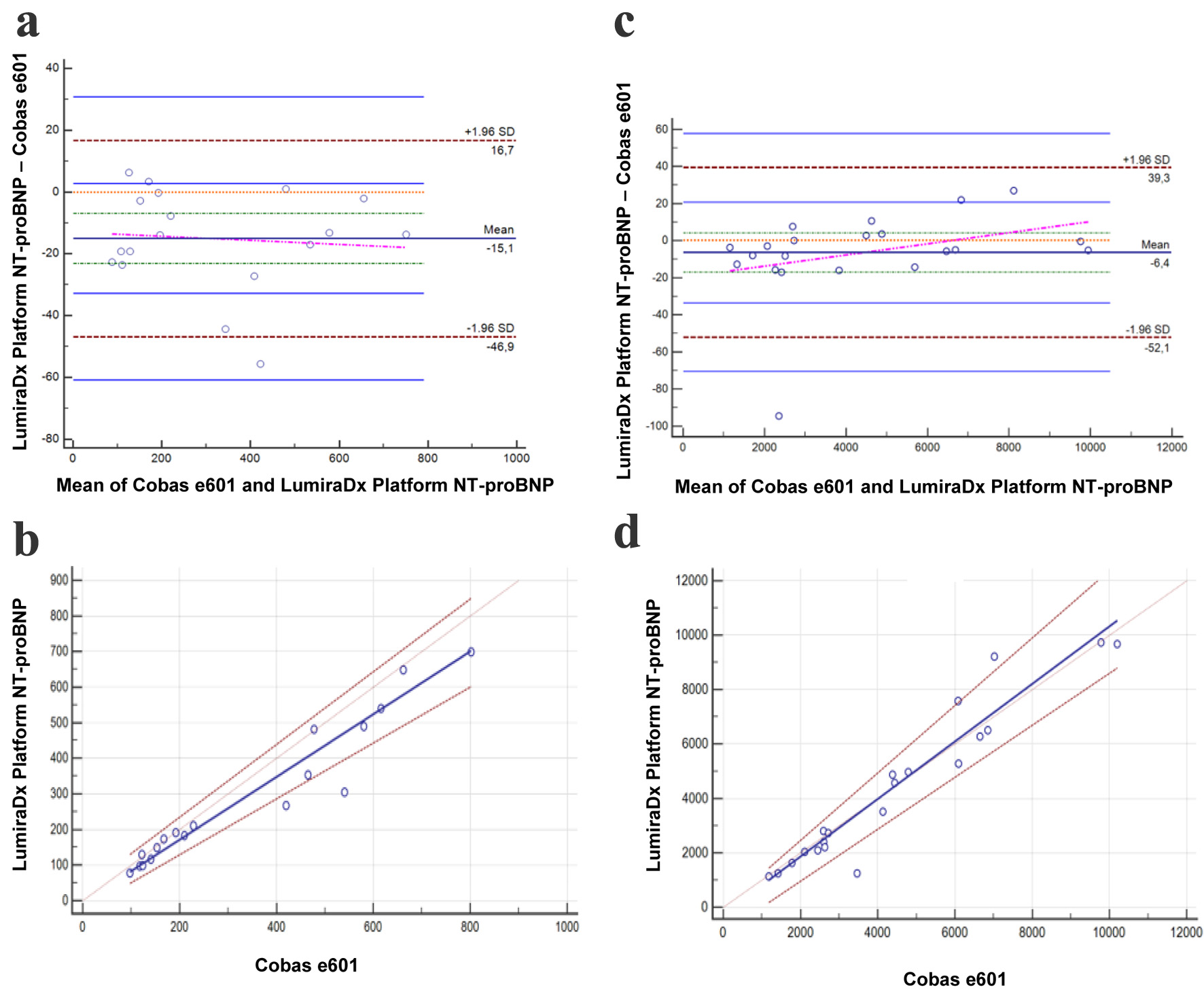 Click for large image | Figure 2. Bland-Altman and Passing-Bablok for the LumiraDx Platform NT-proBNP assay with the second reagent lot. Bland-Altman (a) and Passing-Bablok (b) of values < 1,000 ng/L, for the NT-proBNP LumiraDx Platform assay compared with the gold standard Cobas e601 assay (n = 18). Bland-Altman (c) and Passing-Bablok (d) of values > 1,000 ng/L, for the NT-proBNP LumiraDx Platform assay compared with the gold standard Cobas e601 assay (n = 21). *The units are ng/L and % for Passing-Bablok and Bland-Altman, respectively. NT-proBNP: N-terminal pro-brain natriuretic peptide. |
 Click to view | Table 1. Summary of Analytical Performance of the LumiraDx® NT-proBNP Assay |
The practicability results showed that users were generally satisfied with the LumiraDx® Platform POCT device, particularly appreciated for its ease of use, even without prior training, and for its clear instructions on test procedures and result presentation. However, users noted two reservations: the number of additional components required (device, cartridges, consumables), and the size of the device, which was judged to be of intermediate suitability for a point-of-care setting (Table 2).
 Click to view | Table 2. User-Friendliness of the LumiraDx Platform NT-proBNP POCT Assay |
| Discussion | ▴Top |
POCT devices for NT-proBNP measurement have demonstrated significant clinical value in managing HF), particularly by providing rapid diagnostic results that can guide timely clinical decisions. Adlbrecht et al demonstrated the efficacy of a POCT device for NT-proBNP, highlighting its strong predictive value for clinical events in patients at cardiovascular risk. Their study, involving 1,203 participants, revealed that NT-proBNP levels were superior to clinical signs and symptoms of HF in predicting outcomes, with high negative predictive values for all-cause (86%), cardiac-related (98%), and HF-related (100%) hospitalizations at 125 ng/L [8].
The Cobas h232 POCT device (Roche Diagnostics) has been evaluated in the literature, providing evidence of its effectiveness. Hex et al demonstrated that the Cobas h232 shows strong correlation with laboratory-based measurements from the Cobas e602, with high user satisfaction reported by general practitioners for its ease of use [9]. Similarly, Ceriello et al confirmed the practicality of the Cobas h232 for HF risk stratification in patients with type 2 diabetes and hypertension, supporting its use in routine clinical practice [10]. In the management of HF, POCT devices have increasingly proven their value by offering rapid diagnostic results and thus significantly reducing TAT compared to centralized laboratory testing. These decentralized systems enable quicker clinical decision-making, which is particularly crucial in the acute and routine management of HF. The potential greatest clinical utility of this platform may lie in the emergency department, where differentiating acute HF from alternative causes of dyspnea remains a major challenge. Rapid NT-proBNP results could accelerate triage and management.
To our knowledge, this is the first study to evaluate the performance of the LumiraDx® NT-proBNP POCT assay, providing new insights into its accuracy and practicality in clinical practice. The method comparison with our laboratory’s reference method (Cobas e601) demonstrated strong concordance. However, the first reagent lot showed a more important bias for values above 1,000 ng/L, with the LumiraDx®. One possible explanation for the bias could be that the epitopes recognized by the two assays are not identical, leading to differences in NT-proBNP detection. Fortunately, this issue has been resolved in the second reagent lot currently under development, where the bias is no longer significant for values above 1,000 ng/L. However, with the second reagent lot, while there is still a bias observed in the Bland-Altman analysis, the Passing-Bablok regression results indicate good overall concordance for values < 1,000 ng/L. It is worth noting that the imprecision for the second reagent lot is slightly above the Westgard threshold. However, the fact that we are well within the stated package insert claims (CV of 9.4% versus 5.4% observed) underlines the robustness of the assay’s performance. Although strong concordance was seen below 1,000 ng/L, accuracy at higher NT-proBNP levels is equally important given that many patients with advanced HF present with values well above this threshold. Underestimation in this range could affect risk stratification and hospitalization decisions.
The user satisfaction survey yielded positive results, indicating that the LumiraDx® Platform is easy to use for healthcare personnel, which is a crucial criterion for a POCT device. Additionally, implementing a decentralized POCT system like LumiraDx® Platform has significantly reduced the TAT for result reporting, with an estimated time savings of 49 min in our tertiary care hospital. An added advantage of this platform is its ability to perform NT-proBNP testing using capillary fingerstick samples, providing a convenient alternative to traditional venous blood collection.
However, one reservation regarding the LumiraDx® Platform system for NT-proBNP is that it is not validated for individuals under 18 years old. This poses a challenge in our pediatric population with HF due to congenital heart defects, where the advantage of capillary sampling is particularly significant as it helps minimize the amount of blood drawn. One limitation of our study is that the number of samples analyzed per generation of lots is relatively small.
The present study did not evaluate the economic aspects of implementing the LumiraDx® NT-proBNP Platform, as this was beyond the scope of our investigation. Future dedicated health-economic studies will be required to assess both the direct costs of testing and the potential savings related to shorter TATs and improved clinical decision-making.
This study has several limitations. First, the sample size was modest (n = 81), and no a priori sample size calculation was performed. Second, samples were derived from a single center, which may limit generalizability. Third, no pediatric validation was performed despite the potential value of capillary sampling in children. Fourth, clinical and demographic details of the population were limited, restricting subgroup analyses. These aspects should be addressed in future, larger multicenter studies. Finally, given the observed differences between reagent lots, continuous post-market surveillance will be essential to ensure long-term reliability, particularly for high NT-proBNP concentrations.
In conclusion, the LumiraDx® Platform NT-proBNP POCT system offers a promising approach for managing HF, with notable benefits in ease of use and TAT. However, the observed biases, particularly at higher NT-proBNP values, underline the importance of consistent and standardized testing methods.
Acknowledgments
We would like to acknowledge LumiraDx for providing the reagents used in this evaluation of the LumiraDx® NT-proBNP point-of-care testing device. The company had no role in the study design, data analysis, or interpretation of the results.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from all patients.
Author Contributions
FB, MB, AL, TL, and LVB contributed to data collection and experimental work. ACP provided clinical expertise and helped with study design and interpretation. DG conceived the study, supervised the project, performed data analysis, and drafted the manuscript. All authors reviewed and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726.
doi pubmed - Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895-e1032.
doi pubmed - Pop-Busui R, Januzzi JL, Bruemmer D, Butalia S, Green JB, Horton WB, Knight C, et al. Heart failure: an underappreciated complication of diabetes. a consensus report of the American Diabetes Association. Diabetes Care. 2022;45(7):1670-1690.
doi pubmed - Clerico A, Passino C, Franzini M, Emdin M. Cardiac biomarker testing in the clinical laboratory: where do we stand? General overview of the methodology with special emphasis on natriuretic peptides. Clin Chim Acta. 2015;443:17-24.
doi pubmed - Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, Pinto YM, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27(3):330-337.
doi pubmed - Bayes-Genis A, Rosano G. Unlocking the potential of natriuretic peptide testing in primary care: A roadmap for early heart failure diagnosis. Eur J Heart Fail. 2023;25(8):1181-1184.
doi pubmed - LumiraDx NT-proBNP insert kit. SPEC-35872 R2 ART-02677 R21 Date of Rev 2022.
- Adlbrecht C, Neuhold S, Hulsmann M, Strunk G, Ehmsen U, Scholten C, Maurer G, et al. NT-proBNP as a means of triage for the risk of hospitalisation in primary care. Eur J Prev Cardiol. 2012;19(1):55-61.
doi pubmed - Hex C, Smeets M, Penders J, Van Hoof V, Verbakel J, Buntinx F, Vaes B. Accuracy, user-friendliness and usefulness of the Cobas h232 point-of-care test for NT-proBNP in primary care. J Clin Pathol. 2018;71(6):539-545.
doi pubmed - Ceriello A, Lalic N, Montanya E, Valensi P, Khunti K, Hummel M, Schnell O. NT-proBNP point-of-care measurement as a screening tool for heart failure and CVD risk in type 2 diabetes with hypertension. J Diabetes Complications. 2023;37(3):108410.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.