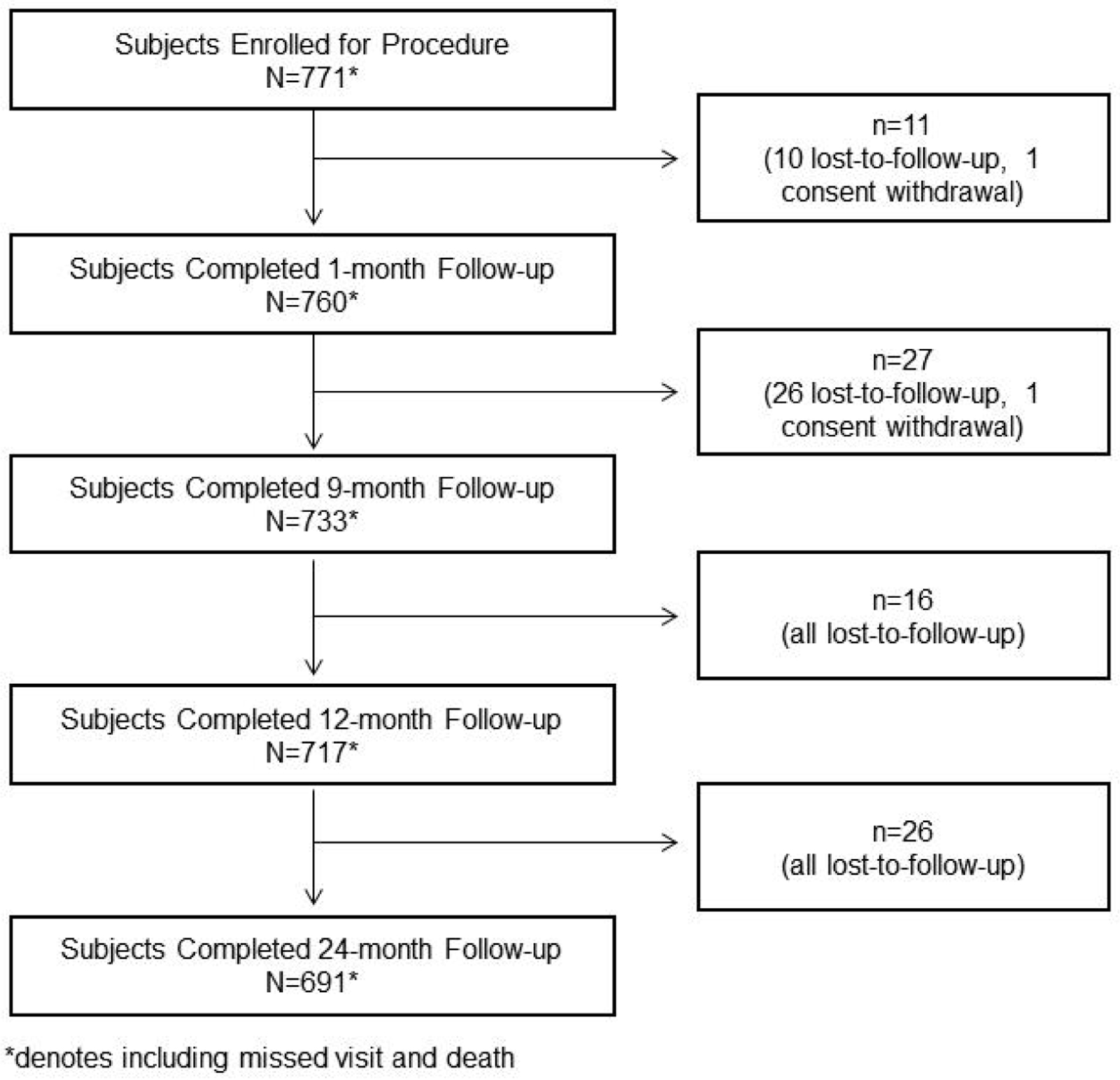
Figure 1. Patient disposition.
| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 15, Number 6, December 2024, pages 439-452
Safety and Efficacy of BioMime Sirolimus-Eluting Stent System in All-Comers Real-World Population With Coronary Artery Stenosis: MILES Global Registry
Figure

Tables
| Parameter | Total sample (N = 771) | Stent length > 30 mm (n = 253) | Stent diameter 4 and 4.5 mm (n = 49) | Subjects with bifurcation lesions (n = 82) | Subjects with CTO (n = 127) |
|---|---|---|---|---|---|
| CTO: chronic total occlusion; SD: standard deviation. | |||||
| Age, years, mean ± SD | 64.15 ± 10.95 | 63.05 ± 11.04 | 64.57 ± 12.42 | 64.87 ± 10.33 | 62.86 ± 10.68 |
| Male, n (%) | 576 (74.71) | 189 (74.70) | 39 (79.59) | 64 (78.05) | 98 (77.17) |
| Body mass index, kg/m2, mean ± SD | 26.84 ± 4.42 | 26.88 ± 4.55 | 27.92 ± 4.11 | 26.60 ± 4.00 | 26.11 ± 4.36 |
| Systolic blood pressure, mm Hg, mean ± SD | 132.54 ± 21.68 | 134.14 ± 22.20 | 134.14 ± 24.21 | 135.23 ± 23.27 | 129.18 ± 22.52 |
| Diastolic blood pressure, mm Hg, mean ± SD | 77.10 ± 12.82 | 76.82 ± 12.76 | 79.55 ± 11.44 | 79.12 ± 13.08 | 76.84 ± 14.91 |
| Heart rate, bpm, mean ± SD | 72.74 ± 12.73 | 70.92 ± 11.82 | 71.63 ± 9.86 | 74.88 ± 13.37 | 72.91 ± 13.65 |
| Medical history, n (%) | n = 771 | n = 253 | n = 49 | n = 82 | n = 127 |
| Family history of cardiovascular disease | 209 (27.11) | 69 (27.27) | 17 (34.69) | 24 (29.27) | 36 (28.35) |
| Smokers | 364 (47.21) | 78 (30.83) | 21 (42.86) | 21 (25.61) | 56 (44.09) |
| Alcoholic | 132 (17.12) | 39 (15.42) | 13 (26.53) | 17 (20.73) | 23 (18.11) |
| Diabetes mellitus | 207 (26.85) | 81 (32.02) | 5 (10.20) | 22 (26.83) | 33 (25.98) |
| Dyslipidemia | 391 (50.71) | 130 (51.38) | 20 (40.82) | 40 (48.78) | 52 (40.94) |
| Anemia | 10 (1.30) | 5 (1.98) | - | 2 (2.44) | 2 (1.57) |
| Liver disease | 14 (1.82) | 3 (1.19) | - | 1 (1.22) | 2 (1.57) |
| Hypertension | 490 (63.55) | 171 (67.59) | 28 (57.14) | 49 (59.76) | 75 (59.06) |
| Renal insufficiency | 36 (4.67) | 16 (6.32) | 3 (6.12) | 5 (6.10) | 4 (3.15) |
| Chronic lung disease | 43 (5.58) | 16 (6.32) | 4 (8.16) | 7 (8.54) | 9 (7.09) |
| Peripheral vascular disease | 30 (3.89) | 13 (5.14) | 5 (10.20) | 3 (3.66) | 2 (1.57) |
| Other illness | 137 (17.77) | 39 (15.42) | 11 (22.45) | 22 (26.83) | 26 (20.47) |
| Cardiovascular history, n (%) | n = 771 | n = 253 | n = 49 | n = 82 | n = 127 |
| History of stroke | 33 (4.28) | 11 (4.35) | 21 (42.86) | 3 (3.66) | 4 (3.15) |
| Cardiovascular disease | 139 (18.03) | 58 (22.92) | 6 (12.24) | 16 (19.51) | 20 (15.75) |
| Transient ischemic attack | 6 (0.78) | 1 (0.40) | 0 (0) | 1 (1.22) | 1 (0.79) |
| History of previous myocardial infarction | 118 (15.3) | 42 (16.60) | 7 (14.29) | 11 (13.41) | 15 (11.81) |
| History of percutaneous coronary intervention | 100 (12.97) | 34 (13.44) | 6 (12.24) | 10 (12.20) | 13 (10.24) |
| History of coronary artery bypass graft surgery | 3 (0.39) | 2 (0.79) | 0 (0) | 1 (1.22) | 0 (0) |
| Others | 48 (6.23) | 13 (5.14) | 3 (6.12) | 3 (3.66) | 5 (3.94) |
| Current angina, n (%) | n = 765 | n = 251 | n = 49 | n = 81 | n = 126 |
| Stable | 235 (30.71) | 78 (31.08) | 14 (28.57) | 25 (30.86) | 25 (19.84) |
| Unstable | 363 (47.45) | 116 (46.22) | 27 (55.10) | 40 (49.38) | 60 (47.62) |
| Not applicable | 167 (21.83) | 57 (22.71) | 8 (16.33) | 16 (19.75) | 41 (32.54) |
| Characteristics | Total sample (N = 771) | Stent length > 30 mm (n = 253) | Stent diameter 4 and 4.5 mm (n = 49) | Subjects with bifurcation lesions (n = 82) | Subjects with CTO (n = 127) |
|---|---|---|---|---|---|
| ACC: American College of Cardiology; AHA: American Heart Association; CTO: chronic total occlusion; TIMI: thrombolysis in myocardial infarction. | |||||
| Total number of treatable lesions, n | 1,079 | 278 | 49 | 91 | 141 |
| Diseased vessel, n (%) | n = 755 | n = 250 | n = 48 | n = 80 | n = 126 |
| Single vessel | 486 (64.37) | 146 (58.40) | 32 (66.67) | 37 (46.25) | 68 (53.97) |
| Double vessel | 183 (24.24) | 67 (26.80) | 9 (18.75) | 26 (32.5) | 40 (31.75) |
| Triple vessel or more | 86 (11.39) | 37 (14.80) | 7 (14.58) | 17 (21.25) | 18 (14.29) |
| Lesion location, n (%) | n = 1,079 | n = 278 | n = 49 | n = 91 | n = 141 |
| Left main | 6 (0.56) | 0 (0) | 0 (0) | 2 (2.2) | 0 (0) |
| Right coronary artery | 309 (28.64) | 109 (39.21) | 31 (63.27) | 12 (13.19) | 56 (39.72) |
| Left anterior descending | 458 (42.45) | 117 (42.09) | 17 (34.69) | 51 (56.04) | 47 (33.33) |
| Left circumflex | 169 (15.66) | 43 (15.47) | 1 (2.04) | 19 (20.88) | 24 (17.02) |
| Diagonal | 36 (3.34) | 0 (0) | 0 (0) | 5 (5.49) | 6 (4.26) |
| Obtuse marginal | 54 (5) | 9 (3.24) | 0 (0) | 0 (0) | 4 (2.84) |
| Posterior descending | 17 (1.58) | 0 (0) | 0 (0) | 2 (2.2) | 4 (2.84) |
| Intermediate/anterolateral | 17 (1.58) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Others | 13 (1.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Type of stenosis, n (%) | n = 1,067 | n = 274 | n = 49 | n = 91 | n = 141 |
| De novo | 1,049 (98.31) | 264 (96.35) | 48 (97.96) | 80 (87.91) | 139 (98.58) |
| In-stent | 9 (0.84) | 6 (2.19) | 1 (2.04) | 2 (2.2) | 1 (0.71) |
| Bifurcation | 9 (0.84) | 4 (1.46) | 0 (0) | 9 (9.89) | 1 (0.71) |
| Thrombus load, n (%) | n = 1,067 | n = 269 | n = 49 | n = 91 | n = 141 |
| None | 858 (80.41) | 190 (70.63) | 35 (71.43) | 69 (75.82) | 75 (53.19) |
| Mild | 74 (6.94) | 25 (9.29) | 6 (12.24) | 7 (7.69) | 13 (9.22) |
| Moderate | 95 (8.9) | 40 (14.87) | 5 (10.2) | 10 (10.99) | 26 (18.44) |
| Severe | 40 (3.75) | 14 (5.2) | 3 (6.12) | 5 (5.49) | 27 (19.15) |
| ACC/AHA type, n (%) | n = 1,067 | n = 272 | n = 49 | n = 91 | n = 141 |
| A | 164 (15.37) | 31 (11.4) | 5 (10.2) | 11 (12.09) | 6 (4.26) |
| B1 | 403 (37.77) | 92 (33.82) | 18 (36.73) | 28 (30.77) | 27 (19.15) |
| B2 | 265 (24.84) | 63 (23.16) | 15 (30.61) | 21 (23.08) | 34 (24.11) |
| C | 235 (22.02) | 86 (31.62) | 11 (22.45) | 31 (34.07) | 74 (52.48) |
| Morphology, n (%) | n = 1,064 | n = 273 | n = 49 | n = 91 | n = 141 |
| Concentric | 556 (52.26) | 151 (55.31) | 31 (63.27) | 54 (59.34) | 75 (53.57) |
| Eccentric | 508 (47.74) | 122 (44.69) | 18 (36.73) | 37 (40.66) | 65 (46.43) |
| TIMI flow prior to treatment, n (%) | n = 1,078 | n = 274 | n = 49 | n = 91 | n = 141 |
| TIMI 0 | 157 (14.56) | 62 (22.63) | 5 (10.2) | 14 (15.38) | 118 (83.69) |
| TIMI 1 | 72 (6.68) | 30 (10.95) | 1 (2.04) | 4 (4.4) | 6 (4.26) |
| TIMI 2 | 191 (17.72) | 50 (18.25) | 12 (24.49) | 14 (15.38) | 7 (4.96) |
| TIMI 3 | 658 (61.04) | 132 (48.18) | 31 (63.27) | 59 (64.84) | 10 (7.09) |
| TIMI flow post-procedure, n (%) | n = 1,040 | n = 278 | n = 49 | n = 84 | n = 132 |
| TIMI 0 | 6 (0.58) | 2 (0.72) | 0 (0) | 2 (2.38) | 4 (3.03) |
| TIMI 1 | 5 (0.48) | 1 (0.36) | 0 (0) | 0 (0) | 4 (3.03) |
| TIMI 2 | 25 (2.4) | 5 (1.8) | 2 (4.08) | 0 (0) | 4 (3.03) |
| TIMI 3 | 1,004 (96.54) | 270 (97.12) | 47 (95.92) | 82 (97.62) | 120 (90.91) |
| Total occlusion, n (%) | 141/1,067 (13.21) | 45/101 (44.55) | 48/49 (97.96) | 13/91 (14.29) | 141/141 (100) |
| Tortuosity of lesion, n (%) | n = 1,067 | n = 273 | n = 49 | n = 91 | n = 141 |
| Mild | 784 (73.48) | 197 (72.16) | 43 (87.76) | 66 (72.53) | 104 (73.76) |
| Moderate | 217 (20.34) | 59 (21.61) | 6 (12.24) | 20 (21.98) | 12 (8.51) |
| Severe | 66 (6.19) | 17 (6.23) | 0 (0) | 5 (5.49) | 25 (17.73) |
| Lesion calcification, n (%) | n = 1,067 | n = 273 | n = 49 | n = 91 | n = 141 |
| Mild | 634 (59.42) | 149 (54.58) | 30 (61.22) | 57 (62.64) | 81 (57.45) |
| Moderate | 217 (20.34) | 70 (25.64) | 8 (16.33) | 16 (17.58) | 20 (14.18) |
| Severe | 47 (4.4) | 24 (8.79) | 4 (8.16) | 8 (8.79) | 6 (4.26) |
| Not applicable | 169 (15.84) | 205 (73.74) | 7 (14.29) | 10 (10.99) | 34 (24.11) |
| Procedural characteristics | Total sample (N = 771) | Stent length > 30 mm (n = 253) | Stent diameter 4 and 4.5 mm (n = 49) | Subjects with bifurcation lesions (n = 82) | Subjects with CTO (n = 127) |
|---|---|---|---|---|---|
| CTO: chronic total occlusion; SD: standard deviation. | |||||
| Lesion pre-dilatation done, n (%) | 599 (55.51) | 205 (73.74) | 39 (79.59) | 73 (80.22) | 94 (66.67) |
| Lesion length, mm, mean ± SD, (n = 1,075) | 22.75 ± 12.22 | 34.61 ± 12.72 | 16.04 ± 6.95 | 21.32 ± 10.22 | 25.16 ± 9.49 |
| Stent length, mm, n (%) | n = 1,039 | n = 278 | n = 52 | n = 84 | n = 132 |
| 8 | 10 (0.96) | 0 (0) | 0 (0) | 1 (1.19) | 0 (0) |
| 13 | 82 (7.89) | 0 (0) | 6 (11.54) | 8 (9.52) | 4 (3.03) |
| 16 | 119 (11.45) | 0 (0) | 6 (11.54) | 8 (9.52) | 12 (9.09) |
| 19 | 206 (19.83) | 0 (0) | 26 (50) | 13 (15.48) | 13 (9.85) |
| 24 | 205 (19.73) | 0 (0) | 7 (13.46) | 18 (21.43) | 28 (21.21) |
| 29 | 138 (13.28) | 0 (0) | 4 (7.69) | 10 (11.9) | 19 (14.39) |
| 32 | 84 (8.08) | 84 (30.22) | 2 (3.85) | 9 (10.71) | 18 (13.64) |
| 37 | 64 (6.16) | 63 (22.66) | 1 (1.92) | 1 (1.19) | 13 (9.85) |
| 40 | 63 (6.06) | 63 (22.66) | 0 (0) | 8 (9.52) | 12 (9.09) |
| 44 | 39 (3.75) | 39 (14.03) | 0 (0) | 4 (4.76) | 8 (6.06) |
| 48 | 29 (2.79) | 29 (10.43) | 0 (0) | 4 (4.76) | 5 (3.79) |
| Stent length, mm, mean ± SD | 25.57 ± 9.35 | 38.30 ± 5.24 | 20.25 ± 5.29 | 26.45 ± 10.12 | 29.11 ± 9.21 |
| Stent diameter, mm, n (%) | n = 1,039 | n = 278 | n = 52 | n = 84 | n = 132 |
| 2.5 | 247 (23.77) | 74 (26.62) | 0 (0) | 22 (26.19) | 37 (28.03) |
| 2.75 | 162 (15.59) | 50 (17.99) | 0 (0) | 13 (15.48) | 18 (13.64) |
| 3 | 363 (34.94) | 104 (37.41) | 0 (0) | 26 (30.95) | 48 (36.36) |
| 3.5 | 214 (20.6) | 47 (16.91) | 0 (0) | 19 (22.62) | 28 (21.21) |
| 4 | 38 (3.66) | 2 (0.72) | 37 (71.15) | 4 (4.76) | 0 (0) |
| 4.5 | 15 (1.44) | 1 (0.36) | 15 (28.85) | 0 (0) | 1 (0.76) |
| Stent diameter, mm, mean ± SD (n = 1,039) | 3.00 ± 0.44 | 2.92 ± 0.36 | 4.14 ± 0.23 | 2.99 ± 0.43 | 2.94 ± 0.38 |
| % Diameter stenosis, pre-procedure, mean ± SD | 83.93 ± 12.84 | 83.48 ± 12.37 | 83.12 ± 17.30 | 85.04 ± 12.68 | 99.21 ± 3.74 |
| % Diameter stenosis post-procedure, mean ± SD | 4.22 ± 11.50 | 4.91 ± 8.73 | 4.12 ± 6.90 | 4.64 ± 13.28 | 3.25 ± 10.69 |
| Reference vessel diameter, mm, mean ± SD | 2.96 ± 0.47 | 2.92 ± 0.45 | 3.89 ± 0.70 | 2.93 ± 0.46 | 2.90 ± 0.53 |
| Minimum lumen diameter, pre-procedure, mm, mean ± SD | 1 ± 1.69 | 1.12 ± 2.88 | 1.33 ± 1.00 | 1.08 ± 0.97 | 0.79 ± 1.91 |
| Minimum lumen diameter post-procedure, mm, mean ± SD | 2.96 ± 1.35 | 2.76 ± 0.65 | 3.84 ± 0.49 | 3.09 ±2.75 | 2.88 ± 0.65 |
| Events | In-hospital (n = 771) | 1 month (n = 760) | 9 months (n = 733) | 12 months (n = 717) | 24 months (n = 691) |
|---|---|---|---|---|---|
| Values are n (%). aOne patient suffered from MI, TVR, TVR including TLR. bFour patients suffered from MI, TVR, TVR including TLR. cEight patients suffered from MI, TVR, TVR including TLR. dTen patients suffered from MI, TVR, TVR including TLR. eTwelve patients suffered from MI, TVR, TVR including TLR. f, gAmong 21 patients, fone patient had MI at 1 and 24 months, gone patient had MI at 9 and 24 months. MI: myocardial infarction; TVR: target vessel revascularization; TLR: target lesion revascularization; TVF: target vessel failure (composite of cardiac deaths, MI, and TVR including TLR); MACE: major adverse cardiovascular events (composite endpoint of cardiac death, MI, and TVR). | |||||
| Death | 0 (0) | 1 (0.13) | 6 (0.81) | 8 (1.11) | 14 (2.02) |
| Cardiac death | 0 (0) | 1 (0.13) | 2 (0.27) | 2 (0.28) | 6 (0.87) |
| Non-cardiac death | 0 (0) | 0 (0) | 3 (0.40) | 4 (0.55) | 5 (0.72) |
| Undetermined death | 0 (0.00) | 0 (0.00) | 1 (0.13) | 2 (0.28) | 3 (0.43) |
| MI | 1 (0.13)a | 6 (0.78)b, f | 14 (1.90)c, g | 18 (2.51)d | 21 (3.03)e, f, g |
| TVR | 1 (0.13)a | 5 (0.65)b | 14 (1.90)c | 17 (2.37)d | 23 (3.32)e |
| TVR including TLR | 1 (0.13)a | 5 (0.65)b | 14 (1.90)c | 17 (2.37)d | 23 (3.32)e |
| TLR | 0 (0) | 3 (0.39) | 12 (1.63) | 15 (2.09) | 21 (3.03) |
| Stent thrombosis | 0 (0) | 0 (0) | 1 (0.13) | 2 (0.28) | 2 (0.28) |
| TVF | 1 (0.13) | 8 (1.05) | 23 (3.13) | 29 (4.04) | 39 (5.64) |
| MACE | 1 (0.13) | 8 (1.05) | 23 (3.13) | 29 (4.04) | 39 (5.64) |
| Events | In-hospital (n = 253) | 1 month (n = 248) | 9 months (n = 239) | 12 months (n = 234) | 24 months (n = 228) |
|---|---|---|---|---|---|
| Values are presented in n (%). aOne patient suffered from MI, TVR and TVR including TLR. bFour patients suffered from MI, TVR and TVR including TLR. cFive patients suffered from MI, TVR and TVR including TLR. dSix patients suffered from MI, TVR and TVR including TLR. e, fAmong nine patients, eone patient had MI at 1 and 24 months, fone patient had MI at 9 and 24 months follow-up. MI: myocardial infarction; TVR: target vessel revascularization; TLR: target lesion revascularization; TVF: target vessel failure (composite of cardiac deaths, MI and TVR including TLR); MACE: major adverse cardiovascular events (composite endpoint of cardiac death, MI and TVR). | |||||
| Death | 0 (0) | 0 (0) | 2 (0.84) | 3 (1.38) | 5 (2.19) |
| Cardiac death | 0 (0) | 0 (0) | 1 (0.42) | 1 (0.43) | 3 (1.32) |
| Non-cardiac death | 0 (0) | 0 (0) | 1 (0.42) | 2 (0.85) | 2 (0.88) |
| Undetermined death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| MI | 0 (0) | 1 (0.40)a, e | 6 (2.51)b, f | 7 (2.99)c | 9 (3.95)d, e, f |
| TVR | 0 (0) | 1 (0.40)a | 8 (3.35)b | 9 (3.85)c | 12 (5.26)d |
| TVR including TLR | 0 (0) | 1 (0.40)a | 8 (3.35)b | 9 (3.85)c | 12 (5.26)d |
| TLR | 0 (0) | 1 (0.40) | 8 (3.35) | 9 (3.85) | 12 (5.26) |
| Stent thrombosis | 0 (0) | 0 (0) | 1 (0.42) | 1 (0.43) | 1 (0.44) |
| TVF | 0 (0) | 1 (0.40) | 11 (4.60) | 12 (5.12) | 16 (7.01) |
| MACE | 0 (0) | 1 (0.40) | 11 (4.60) | 12 (5.12) | 16 (7.01) |
| Events | In-hospital (n = 49) | 1 month (n = 49) | 9 months (n = 48) | 12 months (n = 48) | 24 months (n = 48) |
|---|---|---|---|---|---|
| Values are presented in n (%). aOne patient suffered from MI, TVR and TVR including TLR. bOne patient suffered from MI, TVR and TVR including TLR. cTwo patients suffered from MI, TVR and TVR including TLR. dOne same patient had MI at 9 and 24 months follow-up. MI: myocardial infarction; TVR: target vessel revascularization; TLR: target lesion revascularization; TVF: target vessel failure (composite of cardiac deaths, MI and TVR including TLR); MACE: major adverse cardiovascular events (composite endpoint of cardiac death, MI and TVR). | |||||
| Death | 0 (0) | 0 (0) | 1 (2.08) | 1 (2.08) | 4 (8.33) |
| Cardiac death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.08) |
| Non-cardiac death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.08) |
| Undetermined death | 0 (0) | 0 (0) | 1 (2.08) | 1 (2.08) | 2 (4.17) |
| MI | 0 (0) | 0 (0) | 2 (4.17)a, d | 2 (4.17)b | 3 (6.25)c, d |
| TVR | 0 (0) | 0 (0) | 1 (2.08)a | 1 (2.08)b | 2 (4.17)c |
| TVR including TLR | 0 (0) | 0 (0) | 1 (2.08)a | 1 (2.08)b | 2 (4.17)c |
| TLR | 0 (0) | 0 (0) | 1 (2.08) | 1 (2.08) | 2 (4.17) |
| Stent thrombosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| TVF | 0 (0) | 0 (0) | 3 (6.25) | 3 (6.25) | 5 (10.41) |
| MACE | 0 (0) | 0 (0) | 3 (6.25) | 3 (6.25) | 5 (10.41) |
| Events | In-hospital (n = 82) | 1-month (n = 81) | 9-months (n = 79) | 12-months (n = 78) | 24-months (n = 74) |
|---|---|---|---|---|---|
| Values are presented in n (%). aOne patient suffered from MI, TVR and TVR including TLR. bTwo patients suffered from MI, TVR and TVR including TLR. cFour patients suffered from MI, TVR and TVR including TLR. dFour patients suffered from MI, TVR and TVR including TLR. eOne same patient had MI at 1- and 24-month follow-up. MI: myocardial infarction; TVR: target vessel revascularization; TLR: target lesion revascularization; TVF: target vessel failure (composite of cardiac deaths, MI and TVR including TLR); MACE: major adverse cardiovascular events (composite endpoint of cardiac death, MI and TVR). | |||||
| Death | 0 (0) | 0 (0) | 0 (0) | 1 (1.28) | 3 (4.05) |
| Cardiac death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.35) |
| Non-cardiac death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Undetermined death | 0 (0) | 0 (0) | 0 (0) | 1 (1.28) | 2 (2.70) |
| MI | 0 (0) | 1 (1.23)a, e | 3 (3.84)b | 6 (7.69)c | 7 (9.46)d, e |
| TVR | 0 (0) | 2 (2.47)a | 4 (5.06)b | 6 (7.69)c | 7 (9.46)d |
| TVR including TLR | 0 (0) | 2 (2.47)a | 4 (5.06)b | 6 (7.69)c | 7 (9.46)d |
| TLR | 0 (0) | 2 (2.47) | 4 (5.06) | 6 (7.69) | 7 (9.46) |
| Stent thrombosis | 0 (0) | 0 (0) | 1 (1.27) | 1 (1.28) | 1 (1.35) |
| TVF | 0 (0) | 2 (2.46) | 5 (6.32) | 9 (11.53) | 12 (16.21) |
| MACE | 0 (0) | 2 (2.46) | 5 (6.32) | 9 (11.53) | 12 (16.21) |
| Events | In-hospital (n = 127) | 1 month (n = 126) | 9 months (n = 119) | 12 months (n = 117) | 24 months (n = 113) |
|---|---|---|---|---|---|
| Values are presented in n (%). aTwo patients suffered from MI, TVR and TVR including TLR. bThree patients suffered from MI, TVR and TVR including TLR. cThree patients suffered from MI, TVR and TVR including TLR. MI: myocardial infarction; TVR: target vessel revascularization; TLR: target lesion revascularization; TVF: target vessel failure (composite of cardiac deaths, MI and TVR including TLR); MACE: major adverse cardiovascular events (composite endpoint of cardiac death, MI and TVR). | |||||
| Death | 0 (0) | 0 (0) | 2 (1.68) | 2 (1.71) | 2 (1.77) |
| Cardiac death | 0 (0) | 0 (0) | 1 (0.84) | 1 (0.85) | 1 (0.88) |
| Non-cardiac death | 0 (0) | 0 (0) | 1 (0.84) | 1 (0.85) | 1 (0.88) |
| Undetermined death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| MI | 0 (0) | 1 (0.79) | 4 (3.36)a | 5 (4.27)b | 5 (4.42)c |
| TVR | 0 (0) | 0 (0) | 3 (2.52)a | 5 (4.27)b | 5 (4.42)c |
| TVR including TLR | 0 (0) | 0 (0) | 3 (2.52)a | 5 (4.27)b | 5 (4.42)c |
| TLR | 0 (0) | 0 (0) | 3 (2.52) | 5 (4.27) | 5 (4.42) |
| Stent thrombosis | 0 (0) | 0 (0) | 1 (0.84) | 1 (0.85) | 1 (0.88) |
| TVF | 0 (0) | 1 (0.79) | 6 (5.04) | 8 (6.83) | 8 (7.07) |
| MACE | 0 | 1 (0.79) | 6 (5.04) | 8 (6.83) | 8 (7.07) |
| Stents | No. of patients (n) | Clinical follow-up | Cardiac death | Non-cardiac death | MI | TVR | ST | TVF | MACE |
|---|---|---|---|---|---|---|---|---|---|
| aMACE (composite endpoint of cardiac death, MI, and TVR). bMACE (composite of cardiac death, MI, and TLR). cARC defined - probable/definite. dMACE (death, MI, TVR). eMACE (composite of cardiac death, target vessel-related reinfarction, and ischemia-driven target-lesion revascularization). BMS: bare metal stent; EES: everolimus-eluting stent; MACE: major adverse cardiovascular events; MI: myocardial infarction; SES: sirolimus-eluting stent; ST: stent thrombosis; TVF: target vessel failure, TVR: target vessel revascularization; ZES: zotarolimus-eluting stent. | |||||||||
| BioMime SES | 691 | 2 years | 6 (0.87) | 5.5 (0.72) | 21 (3.03) | 23 (3.32) | 2 (0.28) | 39 (5.64) | 39 (5.64)a |
| XIENCE EES [40] | 365 | 2 years | 3 (0.8) | - | 11(3.0) | 7 (1.9) | 6 (1.6) | - | 22 (6.0)b |
| Resolute ZES [41] | 139 | 2 years | 1 (0.7) | 3 (2.2) | 8 (5.8) | 2 (1.4) | 0 (0.0) | 11 (7.9) | 14 (10.1) |
| MiStent SES [42] | 123 | 2 years | 2 (1.7) | 1 (0.8) | 3 (2.5) | 2 (1.7) | 1 (0.0)c | 6 (5.0) | 8 (6.7)d |
| Endeavor Sprint ZES [42] | 61 | 2 years | 1 (1.7) | 0 (0.0) | 3 (5.0) | 5 (8.3) | 1 (1.7)c | 7 (11.7) | 8 (13.3)d |
| Biolimus [43] | 575 | 1 year | 16 (2.9) | - | - | 11 (2.0) | 5 (0.9) | - | 24 (4.3)e |
| Orsiro SES [26] | 1,245 | 2 years | 21 (1.6) | - | 39 (3.2) | 57 (4.7) | 11 (0.9) | 87 (7.1) | 107 (8.6) |
| Gazelle BMS [43] | 582 | 1 year | 20 (3.5) | - | - | 37 (6.5) | 12 (2.1) | - | 49 (8.7)e |