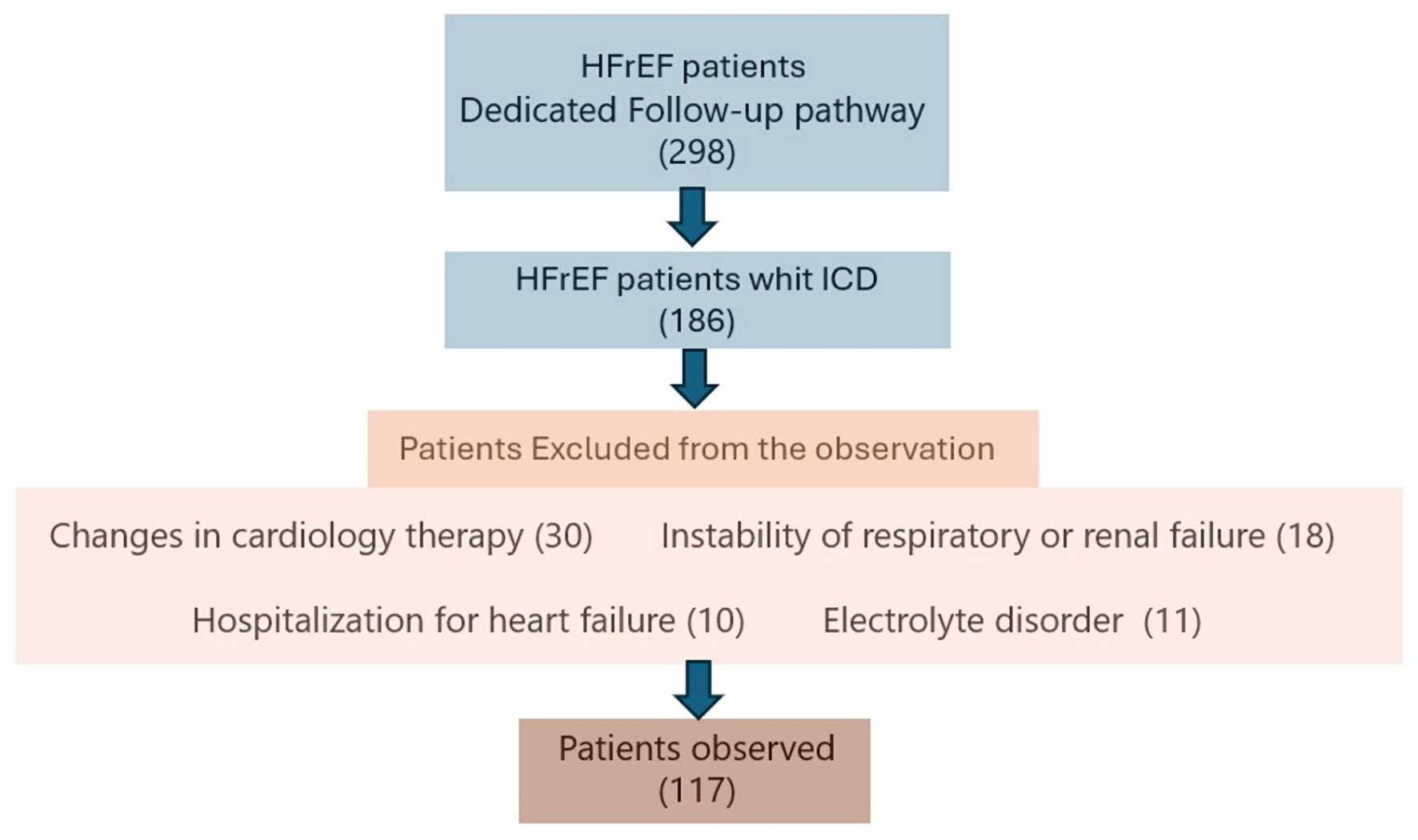
Figure 1. Inclusion and exclusion criteria for the patients’ identification. ICD: implantable cardioverter defibrillator.
| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 2, April 2025, pages 140-152
Effect of Dapagliflozin on Ventricular Arrhythmic Events in Heart Failure Patients With an Implantable Cardioverter Defibrillator
Figures

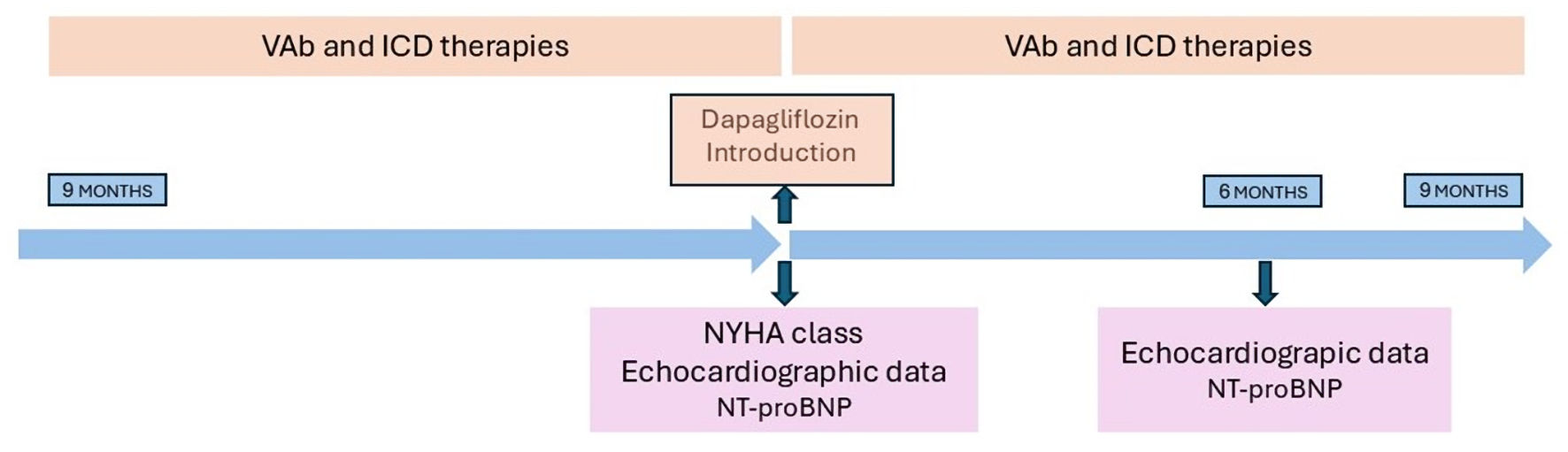
Tables
| N | % | Male, n | Male, % | Female, n | Female, % | |
|---|---|---|---|---|---|---|
| ICD: implantable cardioverter defibrillator; NYHA: New York Heart Association; HTN: hypertension; ICM: ischemic cardiomyopathy; NICM non-ischemic cardiomyopathy; DM: diabetes mellitus; AFib-c: chronic atrial fibrillation; AFib-p: paroxysmal atrial fibrillation; ICD-dc: dual-chamber implantable cardioverter defibrillator; ICD-biv: biventricular implantable cardioverter defibrillator; ICD-sc: single-chamber cardioverter defibrillator; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; ACEI: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; MRA: mineralocorticoid receptor antagonists; APT antiplatelet therapy. | ||||||
| Demographic and clinical data | ||||||
| Age, years | 70.3 ± 11.4 | |||||
| NYHA I/II | 62 | 52.4 | ||||
| NYHA III/IV | 55 | 47.3 | ||||
| Women | 36 | 30.8 | ||||
| Etiology and comorbidity | ||||||
| NICM | 67 | 57.3 | 45 | 55.6 | 22 | 61.1 |
| ICM | 50 | 42.7 | 36 | 44.4 | 14 | 38.9 |
| HTN | 79 | 67.4 | 57 | 70.4 | 22 | 61.0 |
| DM | 35 | 29.9 | 24 | 29.6 | 11 | 30.5 |
| AFib-c | 17 | 14.5 | 7 | 8.6 | 10 | 27.7 |
| AFib-p | 21 | 17.9 | 17 | 21.0 | 4 | 11.1 |
| ICD-dc | 30 | 25.6 | 23 | 28.4 | 7 | 19.4 |
| ICD-biv | 65 | 55.5 | 45 | 55.5 | 20 | 55.5 |
| ICD-sc | 22 | 18.8 | 14 | 17.2 | 8 | 22.2 |
| Ablate and Pace | 7 | 6.0 | 3 | 3.7 | 4 | 11.1 |
| COPD | 21 | 17.9 | 14 | 17.3 | 7 | 19.4 |
| CKD | 29 | 24.8 | 21 | 25.9 | 8 | 22.2 |
| Pharmacotherapy | ||||||
| ACEI/ARBs | 40 | 34.1 | ||||
| ARNI | 71 | 60.6 | ||||
| Beta blocker | 111 | 94.8 | ||||
| MRA | 72 | 61.5 | ||||
| Furosemide | 82 | 70.0 | ||||
| Digoxin | 10 | 8.5 | ||||
| Amiodarone | 30 | 26.2 | ||||
| Statin | 69 | 59.4 | ||||
| Anticoagulants | 38 | 32.3 | ||||
| APT | 71 | 61.2 | ||||
| All | NYHA I/II | NYHA III/IV | P | |
|---|---|---|---|---|
| NYHA: New York Heart Association; LVEF: left ventricular ejection fraction; GLS: global longitudinal strain; E/E’; ventricular filling index; PASP: pulmonary arterial systolic pressure; eGFR: estimated glomerular filtration rate; NT-proBNP: N-terminal B-type natriuretic peptide. | ||||
| Echocardiographic data | ||||
| LVEF, % | 25.9 ± 2.3 | 26.8 ± 1.3 | 24.9 ± 3.7 | 0.01 |
| GLS, % | -12.6 ± 0.9 | -12.3 ± 1.1 | -12.3 ± 1.9 | 0.30 |
| E/E’ | 15.2 ± 2.3 | 14.4 ± 0.6 | 16.2 ± 2.3 | 0.03 |
| PASP, mm Hg | 37.9 ± 3.9 | 35.7 ± 1.8 | 40.5 ± 3.6 | 0.01 |
| Laboratory tests | ||||
| Creatinine, mg/dL | 1.19 ± 0.4 | 1.11 ± 0.6 | 1.21 ± 0.5 | 0.41 |
| eGFR, mL/min/1.73m2 | 71.1 ± 20 | 74.1 ± 23 | 64.1 ± 30 | 0.03 |
| Hemoglobin, g/dL | 12.9 ± 2.4 | 13.1 ± 2.7 | 12.8 ± 2.7 | 0.08 |
| Hematocrit, % | 40 ± 4.6 | 41.1 ± 3.9 | 36.9 ± 4.3 | 0.03 |
| NT-proBNP, pg/L | 1,333.7 ± 564.3 | 1,302.3 ± 272 | 1,368.1 ± 420 | 0.08 |
| Basal | Follow-up | P | |
|---|---|---|---|
| LVEF: left ventricular ejection fraction; GLS: global longitudinal strain; E/E’; ventricular filling index; PASP: pulmonary arterial systolic pressure; NT-proBNP: N-terminal B-type natriuretic peptide. | |||
| LVEF, % | 25.9 ± 2.3 | 28,7 ± 3,6 | 0.025 |
| GLS, % | -12.6 ± 0.9 | -14.7 ± 1.2 | 0.03 |
| E/E’ | 15.2 ± 2.3 | 13.1 ± 1.1 | 0.035 |
| PASP, mm Hg | 37.9 ± 3.9 | 34.1 ± 3.2 | 0.064 |
| NT-proBNP, pg/L | 1,333.74 ± 564.3 | 1,136.1 ± 629.5 | 0.02 |
| Before DAPA | After DAPA | P | |
|---|---|---|---|
| ICD: implantable cardioverter defibrillator; VTA: total ventricular arrhythmias; sVT: sustained ventricular tachycardia; nsVT: non-sustained ventricular tachycardia; VF: ventricular fibrillation; ICDt: ICD therapies. | |||
| VTA | 4.5 ± 2.0 | 2.9 ± 1.8 | 0.01 |
| sVT | 1.2 ± 0.9 | 0.51 ± 0.77 | 0.001 |
| nsVT | 3.84 ± 0.64 | 2.65 ± 1.58 | 0.01 |
| VF | 0.05 ± 0.02 | 0.0 ± 0.0 | 0.11 |
| ICDt | 0.89 ± 1.38 | 0.49 ± 0.76 | 0.014 |
| NYHA I/II | NYHA III/IV | |||||
|---|---|---|---|---|---|---|
| Before DAPA | After DAPA | P | Before DAPA | After DAPA | P | |
| ICD: implantable cardioverter defibrillator; NYHA: New York Heart Association; VTA: total ventricular arrhythmias; sVT: sustained ventricular tachycardia; nsVT: non-sustained ventricular tachycardia; VF: ventricular fibrillation; DAPA: dapagliflozin; ICDt: ICD therapies; ATP: anti-tachycardia pacing. | ||||||
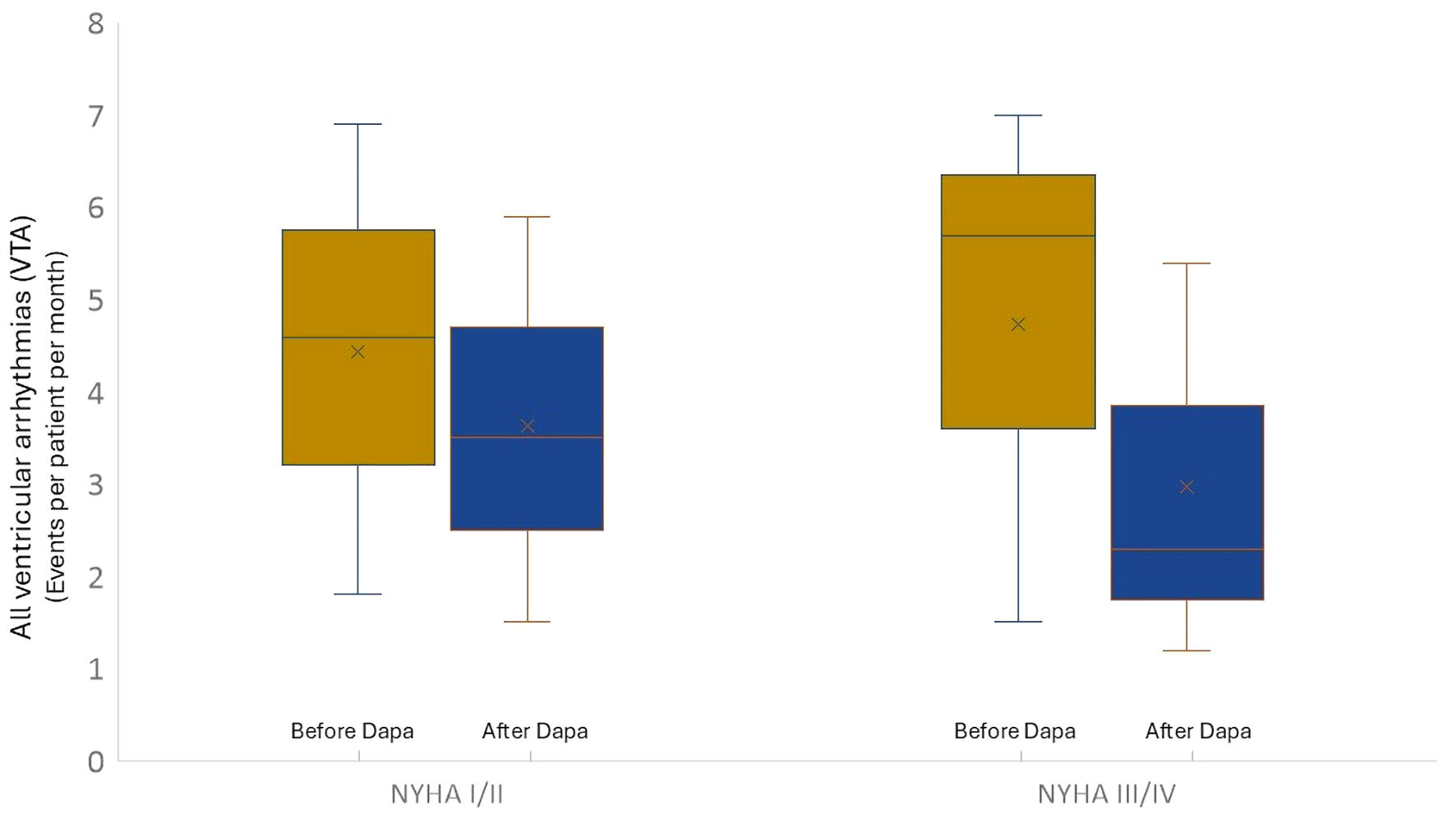
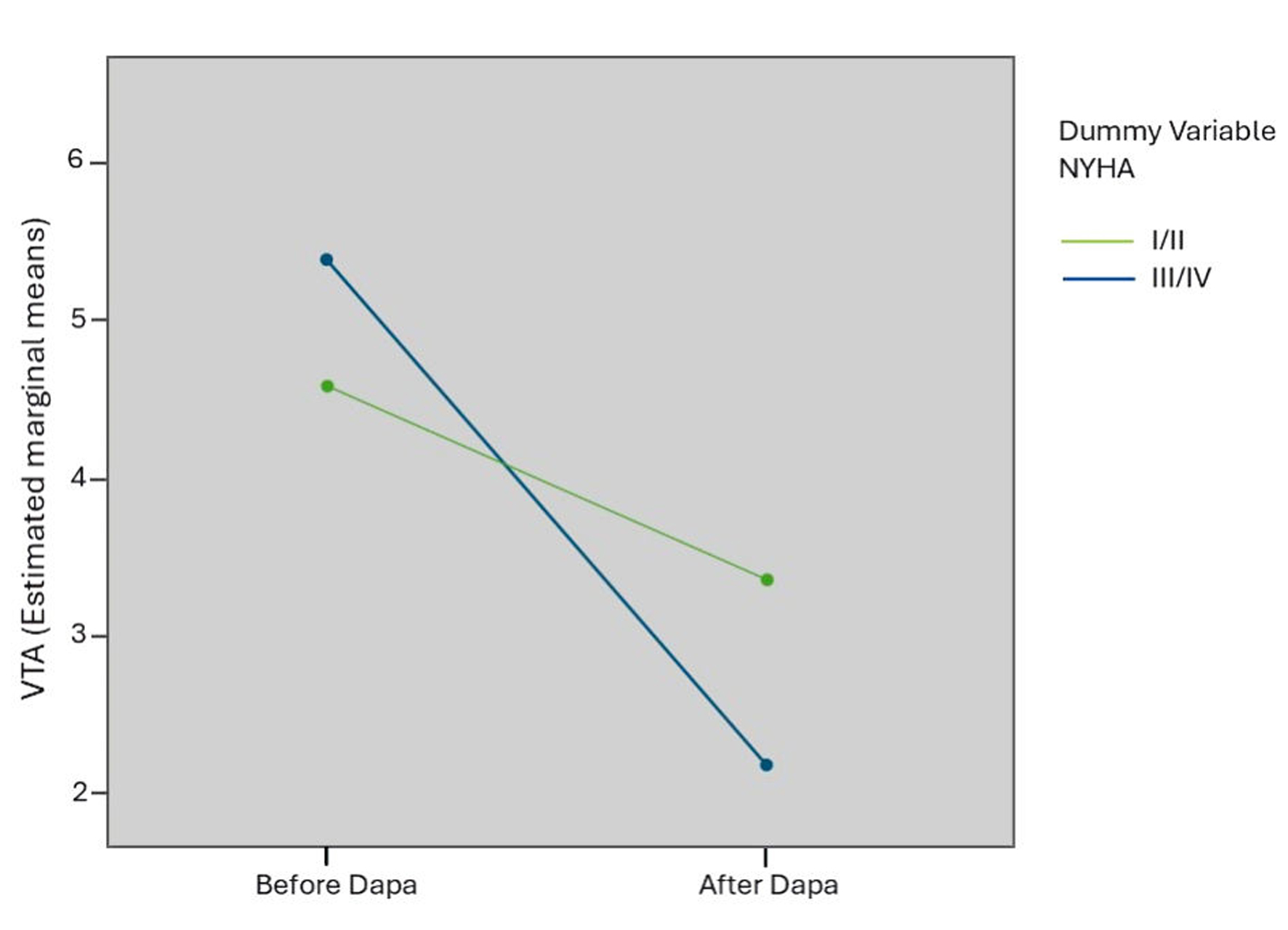
| VTA | 4.5 ± 2.2 | 3.5 ± 2.1 | 0.02 | 5.5 ± 1.8 | 2.2 ± 0.8 | 0.001 |
| sVT | 0.7 ± 0.2 | 0.6 ± 0.1 | NS | 1.9 ± 0.3 | 0.4 ± 0.2 | 0.02 |
| nsVT | 3.9 ± 1.0 | 2.9 ± 0.9 | 0.02 | 3.9 ± 1.2 | 1.7 ± 1.0 | 0.01 |
| VF | 0.0 ± 0.0 | 0.0 ± 0.0 | NS | 0.2 ± 0.1 | 0.0 ± 0.0 | NS |
| ICDt | 069 ± 0,33 | 0.52 ± 0.41 | NS | 1.1 ± 1.08 | 0.66 ± 0.46 | 0.02 |
| ICD-sc | 0.1 ± 0.0 | 0.0 ± 0.0 | NS | 0.2 ± 0.1 | 0.1 ± 0.0 | NS |
| ATP | 0.6 ± 0.2 | 0.6 ± 0.1 | NS | 1.5 ± 0.6 | 0.4 ± 0.2 | 0.003 |
| Δ LVEF < 15% | ΔLVEF > 15% | |||||
|---|---|---|---|---|---|---|
| Before DAPA | After DAPA | P | Before DAPA | After DAPA | P | |
| ICD: implantable cardioverter defibrillator; VTA: total ventricular arrhythmias; sVT: sustained ventricular tachycardia; nsVT: non-sustained ventricular tachycardia; VF: ventricular fibrillation; DAPA: dapagliflozin; ICDt: ICD therapies; ICD-sc: single-chamber cardioverter defibrillator; ATP: anti-tachycardia pacing. | ||||||
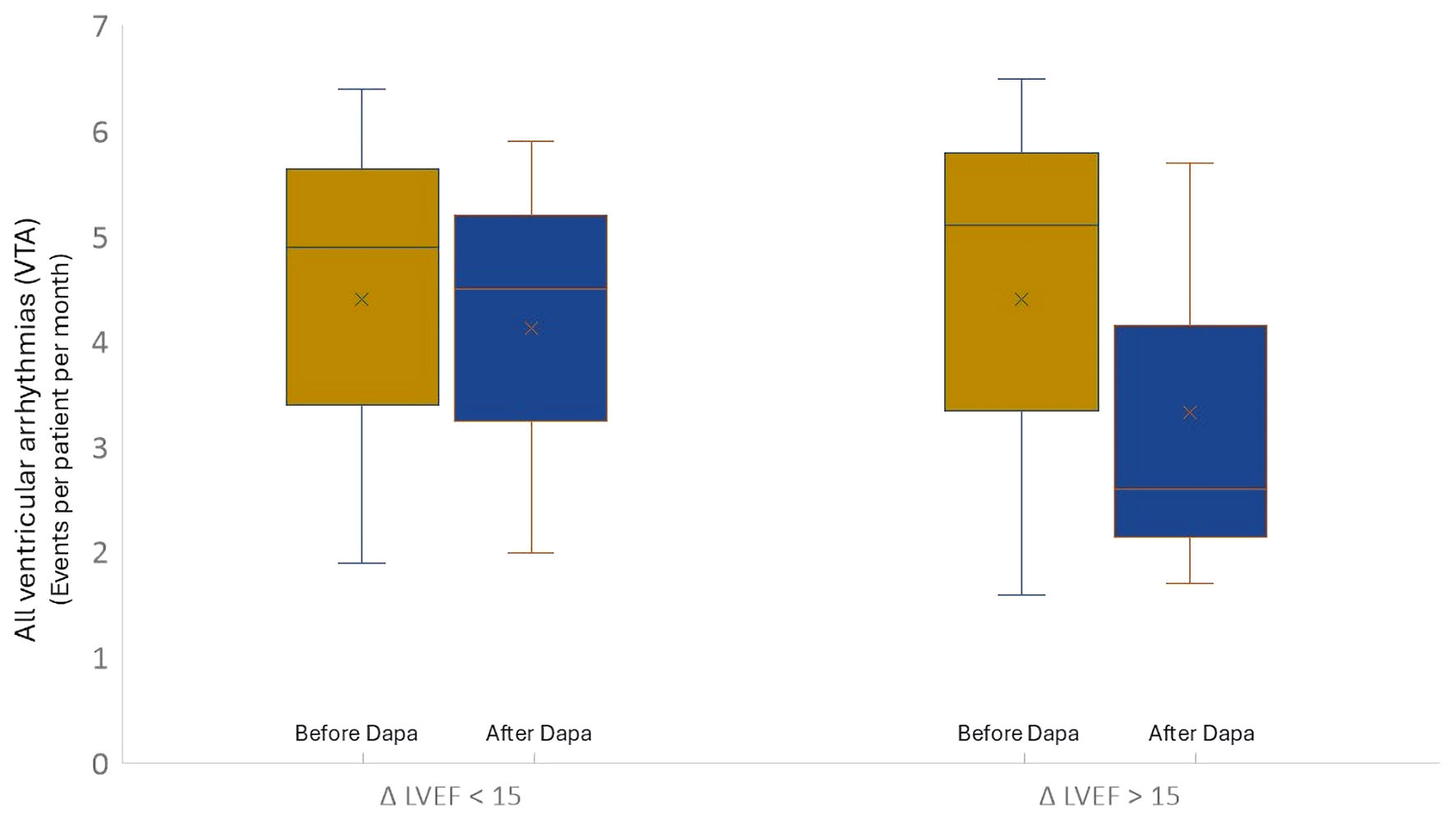
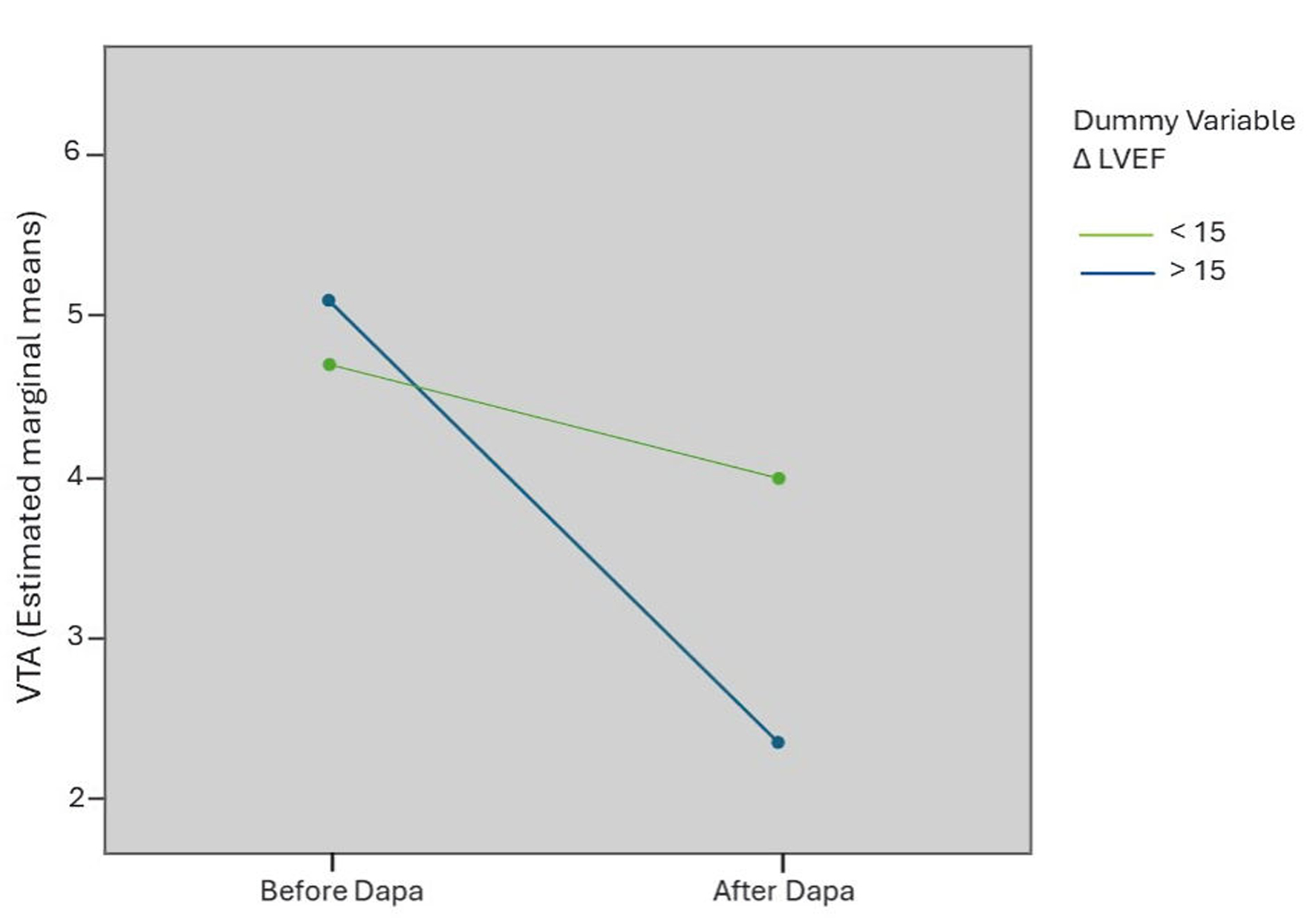
| VTA | 4.9 ± 2.1 | 4.5 ± 2.1 | NS | 5.1 ± 1.6 | 2.5 ± 1.1 | 0.01 |
| sVT | 0.9 ± 0.3 | 0.5 ± 0.2 | NS | 1.2 ± 0.3 | 0.8 ± 0.2 | 0.02 |
| nsVT | 3.4 ± 1.1 | 2.9 ± 1.0 | 0.03 | 3.9 ± 1.5 | 1.8 ± 1.1 | 0.002 |
| VF | 0.0 ± 0.0 | 0.0 ± 0.0 | NS | 0.2 ± 0.0 | 0.0 ± 0.0 | NS |
| ICDt | 0.58 ± 0.31 | 0.53 ± 0.40 | NS | 1.0 ± 1.1 | 0.77 ± 0.42 | 0.02 |
| ICD-sc | 0.0 ± 0.0 | 0.0 ± 0.0 | NS | 0.2 ± 0.1 | 0.0 ± 0.0 | NS |
| ATP | 0.7 ± 0.2 | 0.6 ± 0.2 | NS | 1.4 ± 0.3 | 0.9 ± 0.2 | 0.001 |
| Male | Female | Gender interaction | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | P | Before | After | P | P/η2 | |||||||||||||||||||||||||||||||||
| VTA | 4.7 ± 2.7 | 3.7 ± 2.9 | 0.025 | 4.9 ± 2.0 | 3.7 ± 2.9 | 0.02 | 0.25/0.021 | ||||||||||||||||||||||||||||||||
| ICDt | 0.54 ± 0.7 | 0.40 ± 0.3 | 0.02 | 0.51 ± 0.7 | 0.43 ± 0.5 | 0.02 | |||||||||||||||||||||||||||||||||
| Diabetic | Non-diabetic | Diabetes interaction | |||||||||||||||||||||||||||||||||||||
| Before | After | P | Before | After | P | P/η2 | |||||||||||||||||||||||||||||||||
| VTA | 4.6 ± 1.9 | 3.1 ± 2.3 | 0.02 | 4.7 ± 1.8 | 3.3 ± 2.4 | 0.025 | 0.20/0.039 | ||||||||||||||||||||||||||||||||
| ICDt | 0.79 ± 1.3 | 0.47 ± 0.7 | 0.01 | 0.69 ± 1.4 | 0.39 ± 0.5 | 0.018 | |||||||||||||||||||||||||||||||||
| ICM | NICM | ICM/NICM interaction | |||||||||||||||||||||||||||||||||||||
| Before | After | P | Before | After | P | P/η2 | |||||||||||||||||||||||||||||||||
| VTA | 5.3 ± 1.2 | 2.9 ± 1.9 | 0.01 | 4.4 ± 2.1 | 3.1 ± 2.1 | 0.02 | 0.20/0.033 | ||||||||||||||||||||||||||||||||
| ICDt | 0.49 ± 0.9 | 0.38 ± 0.3 | 0.025 | 0.51 ± 0.7 | 0.43 ± 0.6 | 0.02 | |||||||||||||||||||||||||||||||||
| Amiodarone | No amiodarone | Amiodarone interaction | |||||||||||||||||||||||||||||||||||||
| Before | After | P | Before | After | P | P/η2 | |||||||||||||||||||||||||||||||||
| VTA | 4.4 ± 2.1 | 3.1 ± 2.4 | 0.02 | 5.0 ± 1.5 | 3.5 ± 2.5 | 0.015 | 0.15/0.043 | ||||||||||||||||||||||||||||||||
| ICDt | 0.56 ± 0.8 | 0.44 ± 0.3 | 0.03 | 0.69 ± 0.7 | 0.57 ± 0.6 | 0.025 | |||||||||||||||||||||||||||||||||
| GDMT | Suboptimal GDMT | GDMT interaction | |||||||||||||||||||||||||||||||||||||
| Before | After | P | Before | After | P | P/η2 | |||||||||||||||||||||||||||||||||
| ICD: implantable cardioverter defibrillator; VTA: total ventricular arrhythmias; ICDt: ICD therapies; ICM: ischemic cardiomyopathy; NICM non-ischemic cardiomyopathy; GDMT: guideline- directed medical therapy. | |||||||||||||||||||||||||||||||||||||||
| VTA | 4.3 ± 2.0 | 2.7 ± 1.6 | 0.015 | 4.9 ± 1.4 | 4.2 ± 2.4 | 0.035 | 0.03/0.184 | ||||||||||||||||||||||||||||||||
| ICDt | 0.51 ± 0.8 | 0.40 ± 0.4 | 0.02 | 0.57 ± 0.7 | 0.49 ± 0.6 | 0.03 | |||||||||||||||||||||||||||||||||