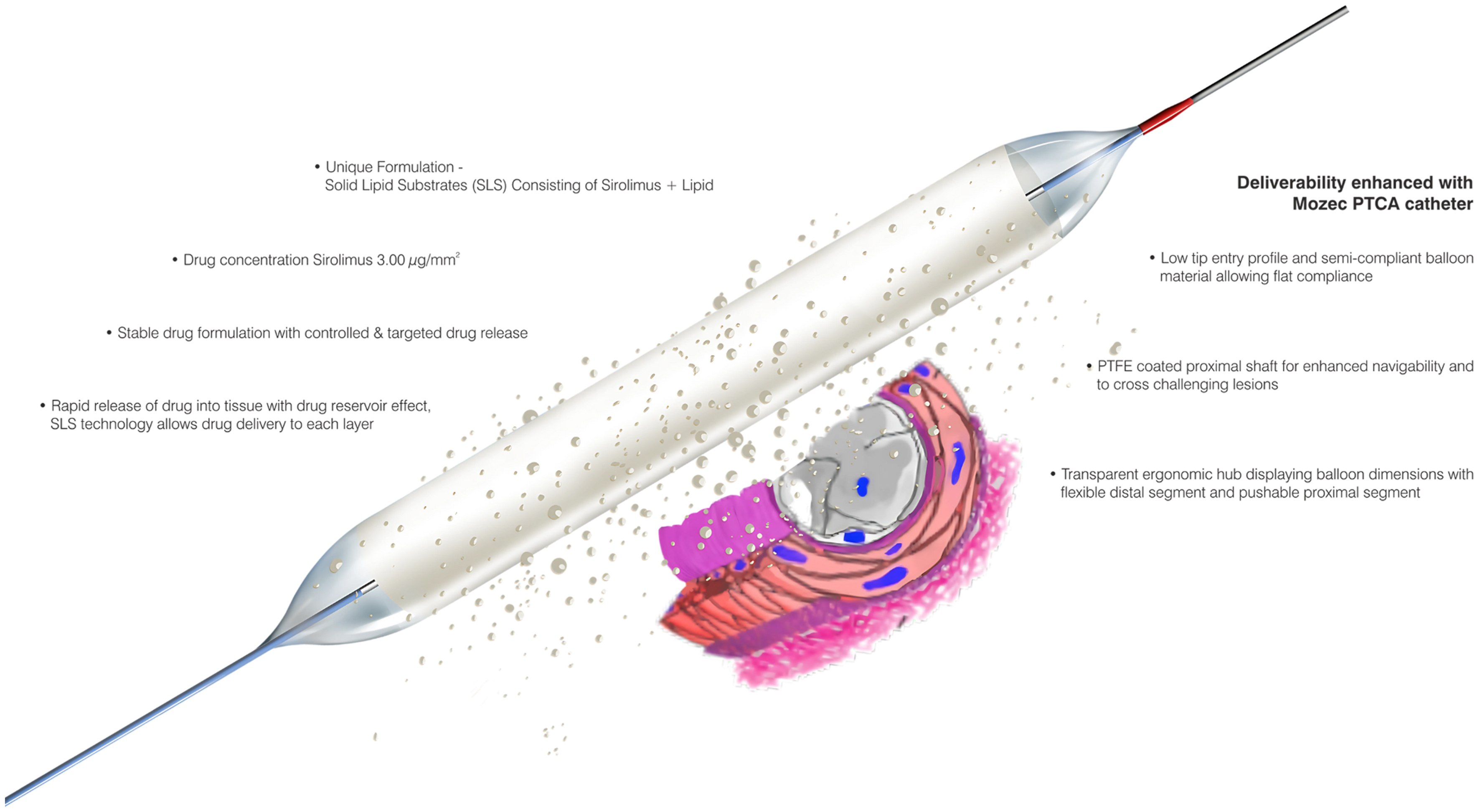
Figure 1. The MOZEC SEB showing its salient features and drug release mechanism. PTFE: polytetrafluoroethylene SEB: sirolimus-eluting balloon.
| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 2, April 2025, pages 130-139
Safety and Performance of the MOZEC Sirolimus-Eluting Coronary Balloon in the Treatment of Stenotic Coronary Artery Lesions: A Real-World, Multicenter, Post-Marketing Surveillance Study
Figures

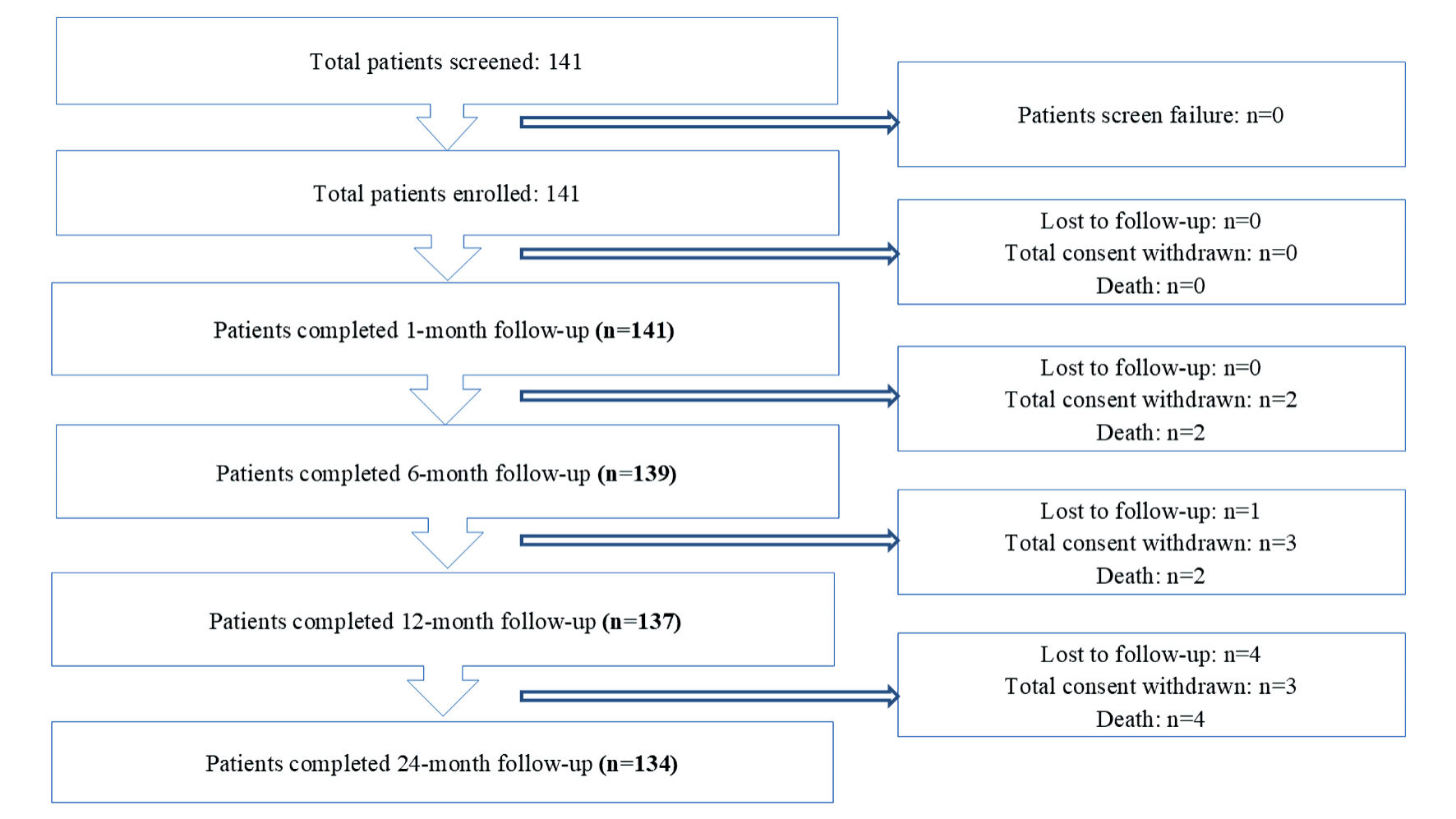
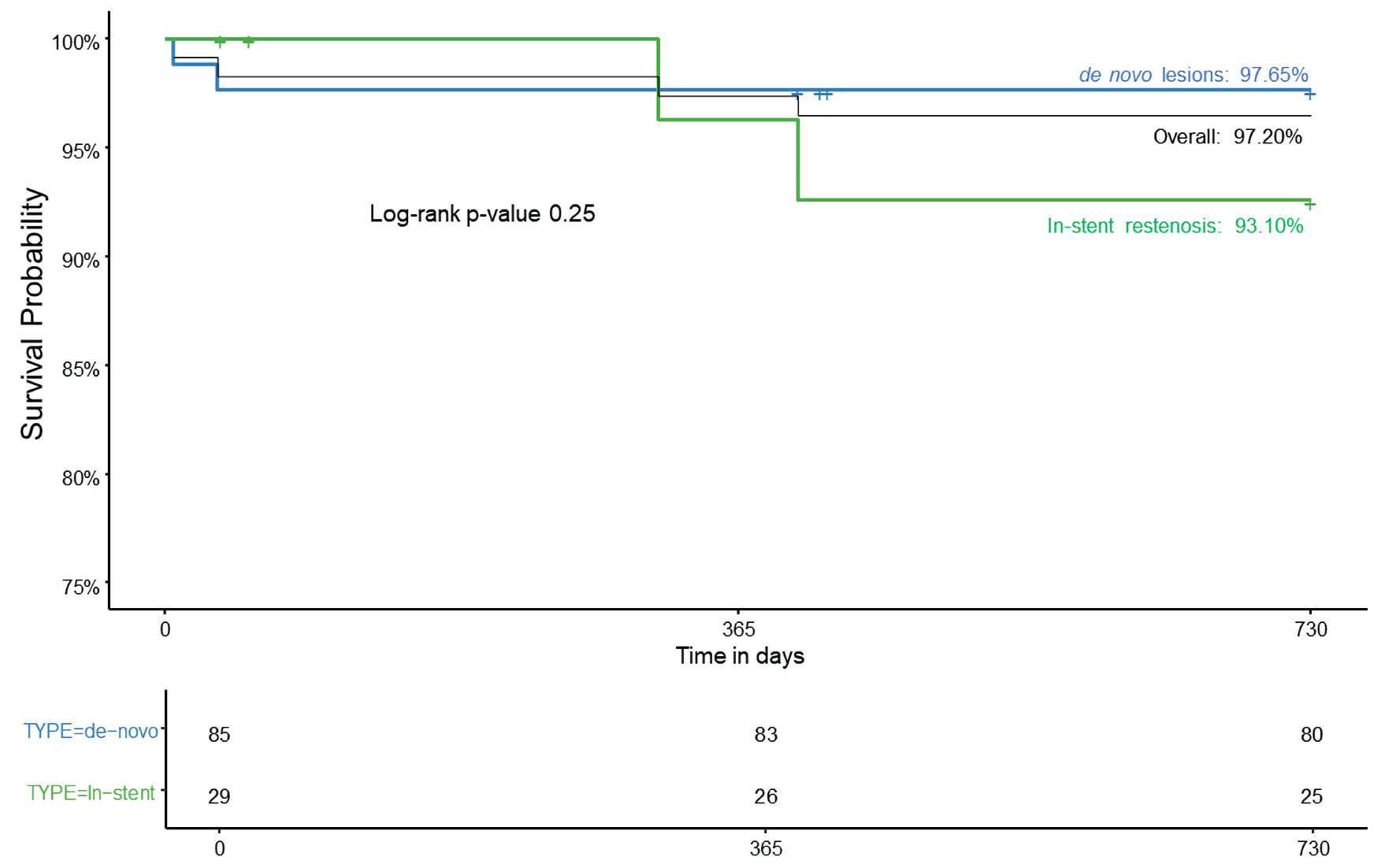
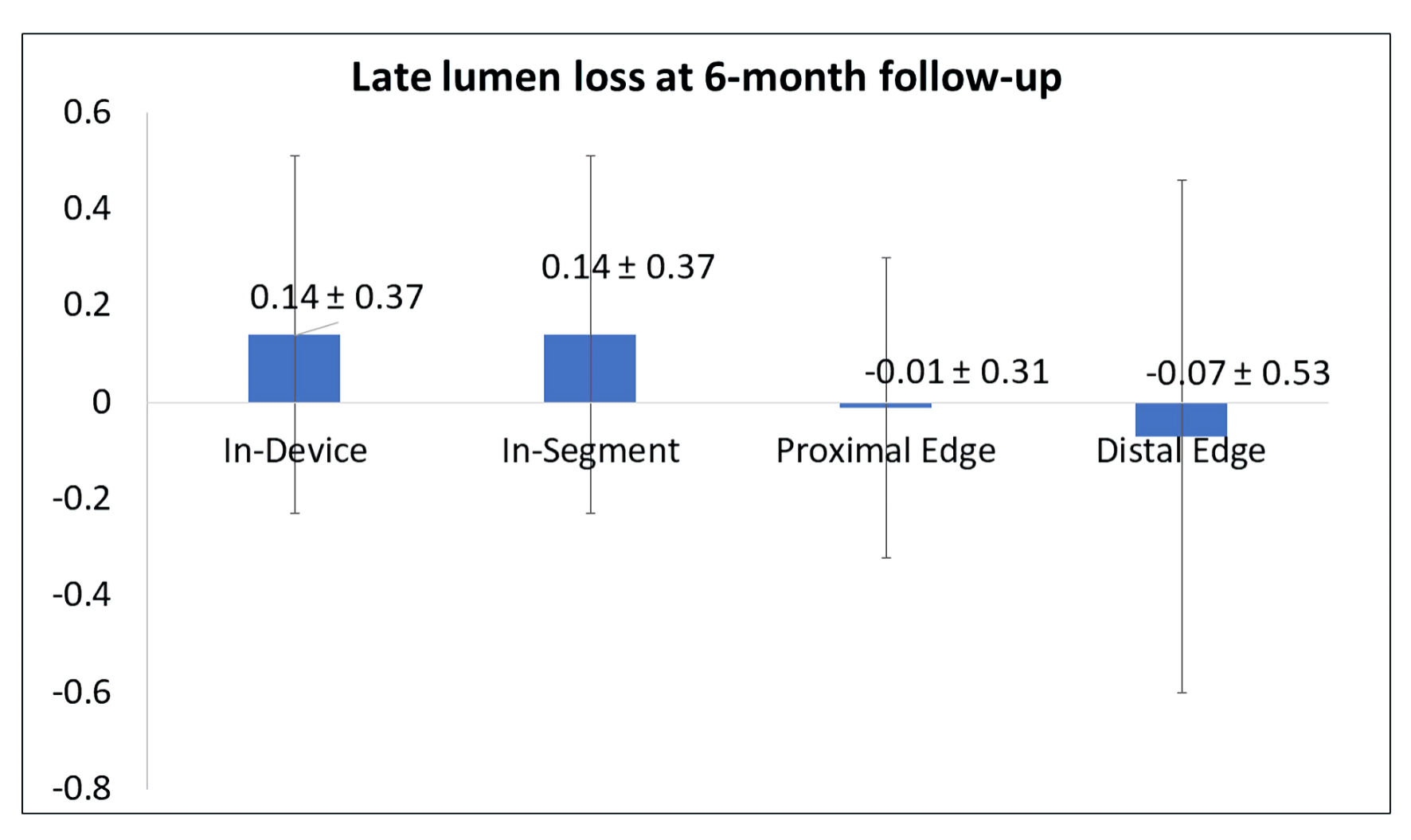
Tables
| Patients (n = 141) | |
|---|---|
| aBronchial asthma, hyperplasia, hypothyroidism and fistula, epigastric hernia and asymptomatic cholelithiasis, non-obstructive hypertrophic cardiomyopathy, interstitial lung disease, obstructive sleep apnea, renal calculi, dyspnea, MI and HBsAg positive. MI: myocardial infraction; CABG: coronary artery bypass graft; CAD: coronary artery disease; HBsAg: hepatitis B surface antigen; PCI: percutaneous coronary intervention; SD: standard deviation. | |
| Characteristics | |
| Age (years), mean ± SD | 59.00 ± 9.84 |
| Male, n (%) | 109 (77.30) |
| Body mass index, kg/m2, mean ± SD | 26.19 ± 3.73 |
| Heart rate, beats/min, mean ± SD | 77.93 ± 11.65 |
| Systolic blood pressure, mm Hg, mean ± SD | 127.43 ± 18.08 |
| Diastolic blood pressure, mm Hg, mean ± SD | 76.19 ± 10.25 |
| Medical history, n (%) | |
| Diabetes mellitus | 85 (60.28) |
| Dyslipidemia | 6 (4.26) |
| Hypertension | 94 (66.67) |
| Chronic renal insufficiency | 1 (0.71) |
| Smokers | 12 (8.51) |
| Alcoholics | 6 (4.26) |
| Other illnessa | 17 (12.06) |
| Cardiac history, n (%) | |
| Previous MI | 27 (19.15) |
| Previous PCI | 40 (28.37) |
| Previous CABG | 6 (4.26) |
| Family history of CAD | 8 (5.67) |
| Diseased coronary arteries, n (%) | |
| Single vessel | 56 (39.72) |
| Double vessels | 41 (29.08) |
| Triple vessels | 42 (29.79) |
| Four vessels | 2 (1.42) |
| Total number of lesions treated | N = 181 |
|---|---|
| LAD: left anterior descending artery; RCA: right coronary artery; LCX: left circumflex coronary artery; LMCA: left main coronary artery; R-PDA: right posterior descending artery; SD: standard deviation. | |
| Lesion’s location, n (%) | N = 181 |
| LAD | 47 (25.96) |
| RCA | 27 (14.91) |
| LCX | 11 (6.07) |
| First diagonal | 21 (11.60) |
| Second diagonal | 1 (0.55) |
| First septal | 1 (0.55) |
| Third obtuse marginal | 2 (1.10) |
| R-PDA | 7 (3.87) |
| Ramus | 5 (2.76) |
| LMCA | 1 (0.55) |
| Others | 58 (32.04) |
| Total number of study devices used to treat the lesion, n | 158 |
| Clinical features of the lesions | |
| Reference vessel diameter (mm), pre-procedure, mean ± SD | 2.53 ± 0.46 |
| Minimum lumen diameter (mm), pre-procedure, mean ± SD | 1.49 ± 0.94 |
| Percentage diameter stenosis, mean ± SD | 86.08 ± 11.02 |
| Lesion length (mm), mean ± SD | 18.40 ± 8.96 |
| Reference vessel diameter (mm), post-procedure, mean ± SD | 2.61 ± 0.46 |
| Minimum lumen diameter (mm), post-procedure, mean ± SD | 2.44 ± 0.51 |
| Events, n (%) | In-hospital (n = 141) | 1-month (n = 141) | 6-month (n = 139) | 12-month (n = 137) | 24-month (n = 134) |
|---|---|---|---|---|---|
| aTwo patients suffered from MI and TLR. bOne patient suffered from MI and cardiac death, cTwo patients suffered from MI and TLR. MACEs: major adverse cardiac events; MI: myocardial infarction; TLR: target lesion revascularization. | |||||
| All-cause death | 0 | 2 (1.42) | 2 (1.44) | 3 (2.19) | 4 (2.99) |
| Cardiac death | 0 | 1 (0.71) | 1 (0.72) | 2 (1.46)b | 2 (1.49) |
| Non-cardiac death | 0 | 1 (0.71) | 1 (0.72) | 1 (0.73) | 2 (1.49) |
| MI | 0 | 1 (0.71) | 3 (2.16)a | 5 (3.65)b, c | 5 (3.73) |
| TLR | 0 | 0 | 2 (1.44)a | 4 (2.92)c | 4 (2.99) |
| Cumulative MACEs | 0 | 2 (1.42) | 4 (2.88) | 6 (4.38) | 6 (4.47) |
| Parameters | Pre-procedure (n = 17) | Post-procedure (n = 17) | 6-month follow-up (n = 17) | P value |
|---|---|---|---|---|
| Values are given in mean ± SD. MLD: minimal lumen diameter; SD: standard deviation. | ||||
| In-device | ||||
| Binary stenosis | 0.93 ± 0.27 | 0.29 ± 0.47 | 0.31 ± 0.48 | 0.0078 |
| MLD (mm) | 0.49 ± 0.37 | 1.33 ± 0.28 | 1.15 ± 0.47 | 0.0005 |
| In-segment | ||||
| Binary stenosis | 0.93 ± 0.27 | 0.29 ± 0.47 | 0.31 ± 0.48 | 0.0078 |
| MLD (mm) | 0.49 ± 0.37 | 1.28 ± 0.25 | 1.15 ± 0.47 | 0.0005 |
| Proximal edge | ||||
| MLD (mm) | 2.04 ± 0.30 | 2.20 ± 0.53 | 2.22 ± 0.50 | 0.2368 |
| Distal edge | ||||
| MLD (mm) | 1.73 ± 0.31 | 1.70 ± 0.34 | 1.82 ± 0.47 | 0.6989 |
| Study name | Study design | Device name | Sample size | Follow-up duration | Cardiac death | MI | TLR | MACE |
|---|---|---|---|---|---|---|---|---|
| aMACEs (composite of cardiac death, MI, and TLR). bMACEs (death, MI, TVR). cMACEs (composite of cardiac death, target vessel-related reinfarction, and ischemia-driven target-lesion revascularization). MACE: major adverse cardiac event; MI: myocardial infarction; SEB: sirolimus-eluting balloon; TLR: target lesion revascularization; SCB: sirolimus-coated balloon; SES: sirolimus-eluting coronary stent system, EES: everolimus-eluting coronary stent system; ZES: zotarolimus-eluting coronary stent system; RCT: randomized controlled trial. | ||||||||
| MOZEC SEB | Real-world, multicenter, post-marketing surveillance study | MOZEC SEB | 141 | 2 years | 1.49% | 3.73% | 2.99% | 4.47% |
| BASKET-SMALL 2 [14] | Multicenter, open-label, randomized noninferiority trial | Paclitaxel-coated balloon SeQuent Please® | 382 | 1 year | 3.1% | 1.6% | - | 7.5% |
| Everolimus-eluting Xience® stent and paclitaxel-eluting TAXUS Element® stent | 376 | 1 year | 1.3% | 3.5% | - | 7.3% | ||
| EASTBOURNE prospective registry [17] | Prospective, multicenter, real-world study | Magic Touch sirolimus DCB | 2,123 | 1 year | 1.5% | 2.4% | 5.9% | 9.9% |
| Nanolute registry final results [19] | Prospective registry | Magic Touch SCB | 408 | 2 years | 0.7% | 0.2% | 3.2% | 4.2% |
| SELFIE registry [21] | Prospective, single-center registry | Magic Touch SCB | 62 | 11 ± 7 months | 1.6% | 3.2% | 3.2% | 4.8% |
| BIONYX trial [25] | Prospective, patient- and assessor-blinded, randomized noninferiority trial | Orsiro SES | 1,245 | 2 years | 1.6% | 3.2% | 3.4% | 8.6% |
| Resolute Onyx SES | 1,243 | 2 years | 1.0% | 3.3% | 3.9% | 8.3% | ||
| THRIVE study [26] | Prospective, multicenter, real-world, single-arm registry | XIENCE EES | 365 | 2 years | 0.8% | 3.0% | 2.1% | 6.0%a |
| RESOLUTE clinical trial [27] | Prospective, multicenter, non-randomized, single-arm trial | Resolute ZES | 139 | 2 years | 0.7% | 5.8% | 1.4% | 10.1% |
| DESSOLVE I and II trials [28] | DESSOLVE I: first-in-human, single-arm trial; and DESSOLVE II: randomized trial | MiStent SES | 123 | 2 years | 1.7% | 2.5% | 1.7% | 6.7%b |
| Endeavor Sprint ZES | 61 | 2 years | 1.7% | 5.0% | 1.7% | 13.3%b | ||
| COMFORTABLE AMI RCT [29] | Prospective, randomized, single-blinded, controlled trial | Biolimus | 575 | 1 year | 2.9% | 2.0% | 1.6% | 4.3%c |