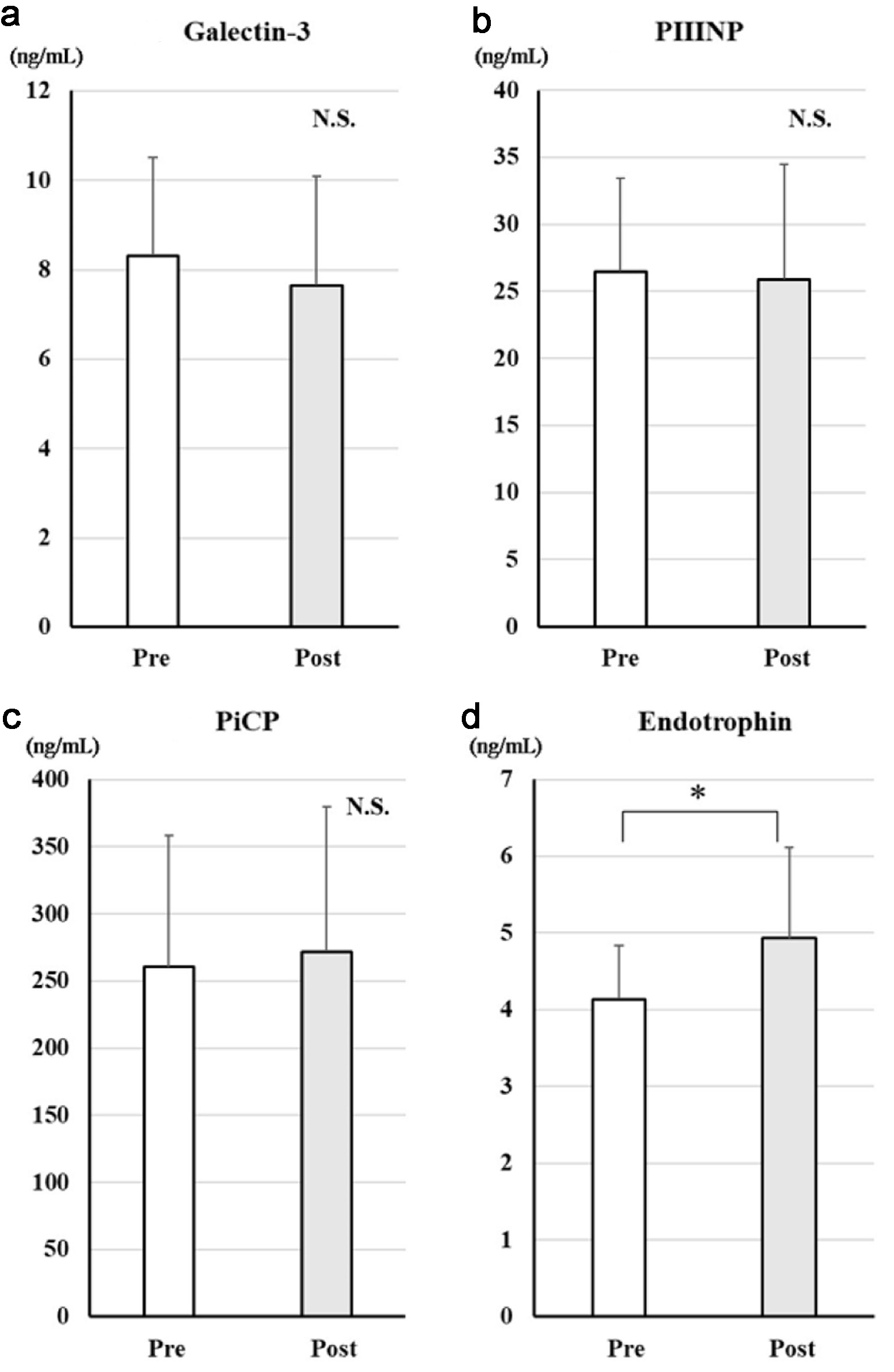
Figure 1. Changes in serum markers of fibrosis after treatment with dapagliflozin for 6 months. Changes in galectin-3 (a), PIIINP (b), PiCP (c), and endotrophin (d) before and after treatment with dapagliflozin. *Significant differences between before and after treatment. PIIINP: procollagen type III N-terminal propeptide; PiCP: procollagen type I C-terminal propeptide; NS: not significant.
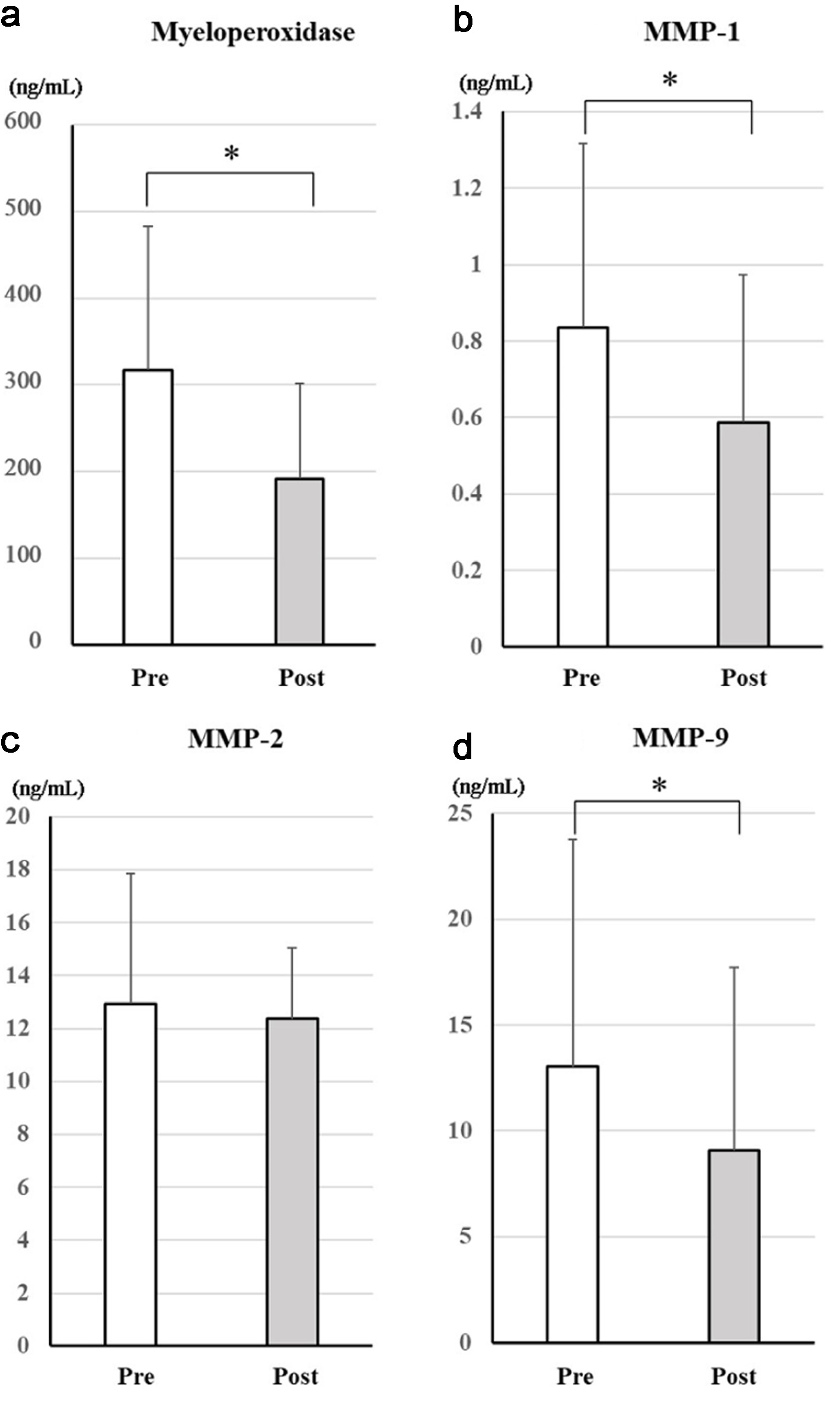
Figure 2. Changes in serum markers of antioxidative effects after treatment with dapagliflozin for 6 months. Changes in myeloperoxidase (a), MMP-1 (b), MMP-2 (c), and MMP-9 (d) between before and after treatment with dapagliflozin. *Significant differences between before and after treatment. MMP-1: matrix metalloproteinase-1; MMP-2: matrix metalloproteinase-2; MMP-9: matrix metalloproteinase-9; NS: not significant.
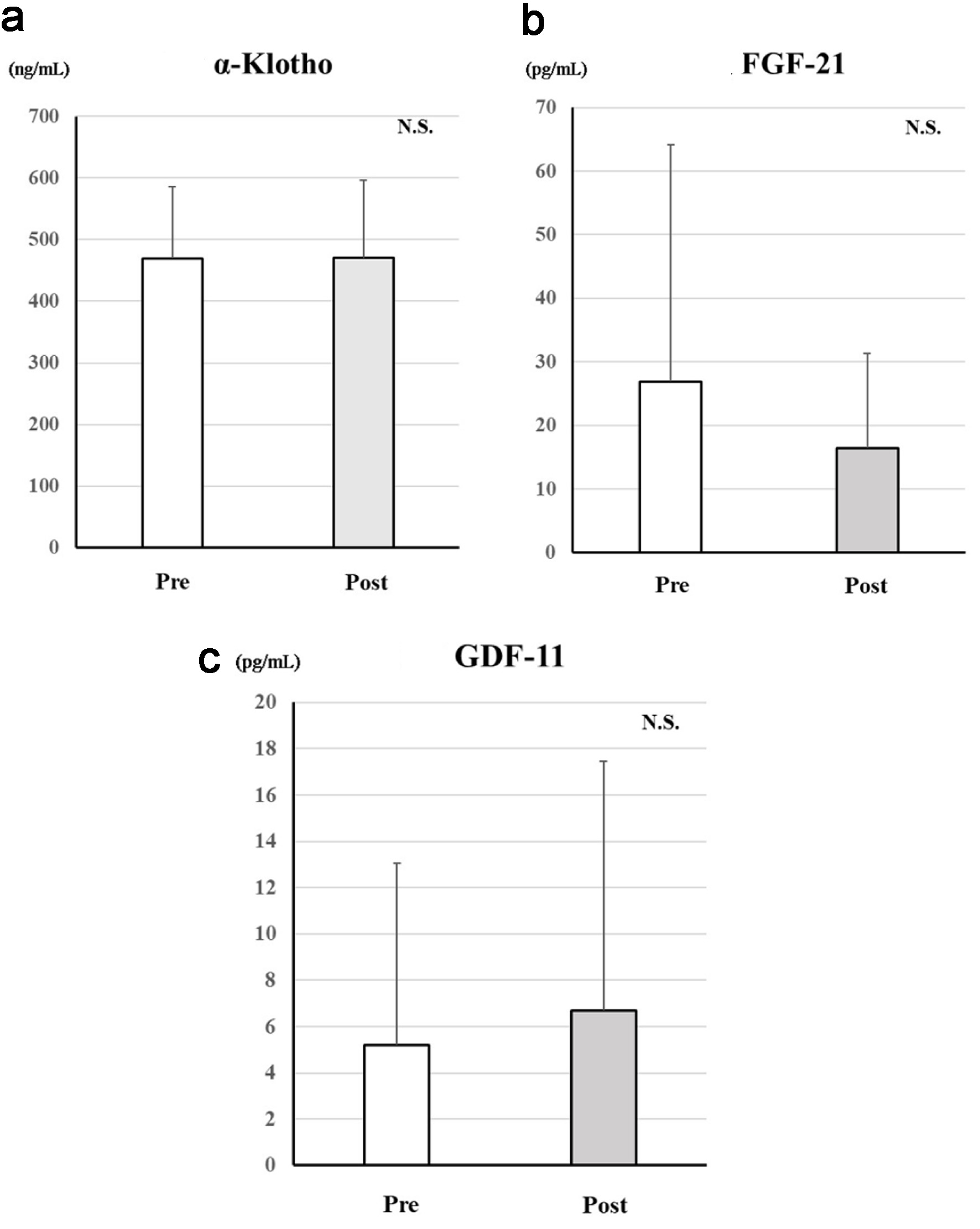
Figure 3. Changes in serum age-related proteins after treatment with dapagliflozin for 6 months. Changes in α-Klotho (a), FGF-21 (b), and GDF-11 (c) between before and after treatment with dapagliflozin. *Significant differences between before and after treatment. FGF-21: fibroblast growth factor-21; GDF-11: growth differentiation factor-11; NS: not significant.


