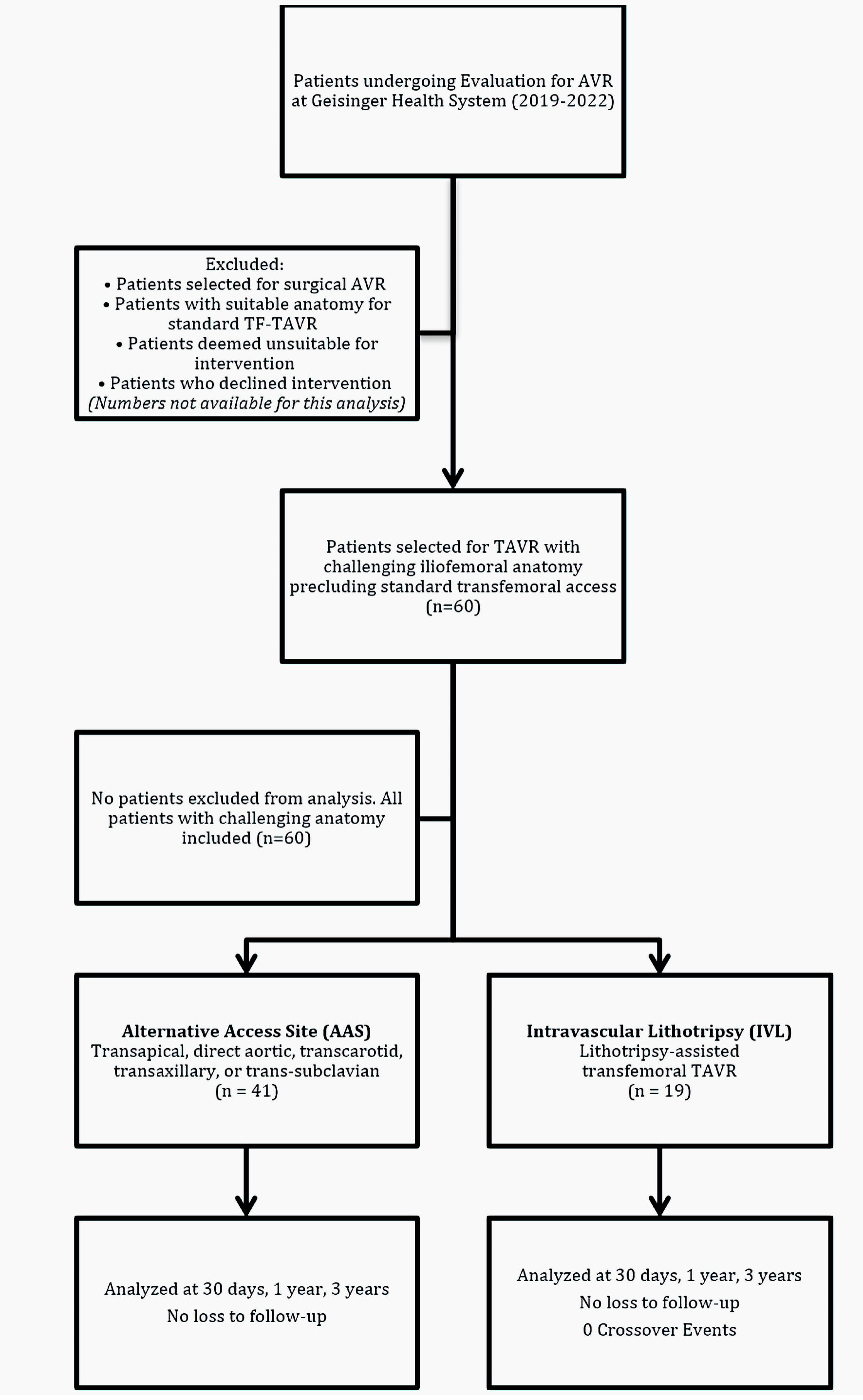
| Demographic information | | | |
| Age, mean (SD) | 77.3 (7.83) | 78.1 (6.92) | 0.69a |
| Gender, n (%) | | | 0.77b |
| Female | 13 (31.7%) | 5 (26.3%) | |
| Male | 28 (68.3%) | 14 (73.7%) | |
| Race, n (%) | | | |
| White | 41 (100.0%) | 19 (100.0%) | |
| Procedure location, n (%) | | | |
| GCMC | 1 (2.4%) | 8 (42.1%) | |
| GMC | 31 (75.6%) | 5 (26.3%) | |
| GWV | 9 (22.0%) | 6 (31.6%) | |
| Outcomes | | | |
| Total length of stay (days), median (IQR) | 3 (2, 6) | 3 (1, 11) | 0.72c |
| Length of stay after procedure (days), median (IQR) | 2 (1, 3) | 2 (1, 3) | 0.37c |
| Adverse outcomes, 1 month, n (%) | | | 0.09b |
| No | 34 (82.9%) | 19 (100%) | |
| Yes | 7 (17.1%) | 0 (0.0%) | |
| Major vascular complication | 1 (2.4%) | 0 (0.0%) | |
| Major bleeding | 2 (4.9%) | 0 (0.0%) | |
| New pacemaker | 2 (4.9%) | 0 (0.0%) | |
| Paravalvular leak | 1 (2.4%) | 0 (0.0%) | |
| Stroke, major vascular complication | 1 (2.4%) | 0 (0.0%) | |
| Adverse outcomes, 1 year, n (%) | | | 0.70b |
| No | 36 (87.8%) | 16 (84.2%) | |
| Yes | 5 (12.2%) | 3 (15.8%) | |
| MI | 2 (4.9%) | 2 (10.5%) | |
| Major bleeding | 1 (2.4%) | 1 (5.3%) | |
| New pacemaker | 1 (2.4%) | 0 (0.0%) | |
| Unknown | 1 (2.4%) | 0 (0.0%) | |
| Repeat hospitalization (30 days), n (%) | | | 0.49b |
| No | 34 (82.9%) | 14 (73.7%) | |
| Yes | 7 (17.1%) | 5 (26.3%) | |
| Acute respiratory failure | 1 (5.3%) | 0 (0.0%) | |
| Aspiration pneumonia | 0 (0.0%) | 1 (10.0%) | |
| Clostridium difficile | 1 (5.3%) | 0 (0.0%) | |
| Chest pain (deemed noncardiac) | 1 (5.3%) | 0 (0.0%) | |
| Dyspnea related to worsening HF | 1 (5.3%) | 0 (0.0%) | |
| Endocarditis | 0 (0.0%) | 1 (10.0%) | |
| Femur fracture from osteoporosis | 0 (0.0%) | 1 (10.0%) | |
| GI bleed | 0 (0.0%) | 1 (10.0%) | |
| Hemorrhagic shock (GI) | 1 (5.3%) | 0 (0.0%) | |
| Pulmonary embolism | 0 (0.0%) | 1 (10.0%) | |
| Sepsis (pneumonia) | 1 (5.3%) | 0 (0.0%) | |
| Pseudoaneurysm from procedure, new AFIB | 1 (5.3%) | 0 (0.0%) | |
| Cause of death, n (%) | | | 0.15d |
| Not cardiovascular | 5 (12.2%) | 0 (0.0%) | |
| Cardiovascular | 3 (7.3%) | 4 (21.1%) | |
| Alive | 31 (75.6%) | 15 (78.9%) | |
| Unknown | 2 (4.9%) | 0 (0.0%) | |