| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 15, Number 6, December 2024, pages 460-466
Effects of Heart Rate Fluctuation on Aerobic Training Outcomes in Patients With Stable Coronary Artery Disease: A Prospective Study
Jun Hong Liua, Hui Hui Songb, Hua Fang Zhanga, Jia Lin Jic, Xue Jiao Zhoud, f, Xi Cai Sune, f
aThe Third Department of Geriatrics, Weifang People’s Hospital, Weifang 261042, Shandong, China
bObstetrics and Gynecology Medical Center, Weifang People’s Hospital, Weifang 261042, Shandong, China
cDepartment of Clinical Medicine, Shandong Second Medical University, Weifang 261053, Shandong, China
dHospital Preparation Center, Weifang People’s Hospital, Weifang 261042, Shandong, China
eHospital Office, Weifang People’s Hospital, Weifang 261042, Shandong, China
fCorresponding Author: Xue Jiao Zhou, Hospital Preparation Center, Weifang People’s Hospital, Weifang 261042, Shandong, China; Xi Cai Sun, Hospital Office, Weifang People’s Hospital, Weifang 261042, Shandong, China
Manuscript submitted August 5, 2024, accepted November 1, 2024, published online November 8, 2024
Short title: Effects of HR Fluctuation on Aerobic Training
doi: https://doi.org/10.14740/cr1710
| Abstract | ▴Top |
Background: This study aimed to evaluate the effects of different heart rate fluctuation ranges during aerobic training on outcomes in patients with stable coronary artery disease (CAD).
Methods: Ninety-seven patients diagnosed with stable CAD were enrolled between March 2017 and December 2019. Participants were randomly assigned to three groups: the control (CON) group, the medium-intensity heart rate small range (MIS) group, and the medium-intensity heart rate large range (MIL) group. The CON group received standard care and patient education, while the MIS and MIL groups underwent personalized rehabilitation training with specific heart rate fluctuation targeted ranges, in addition to standard care. Cardiopulmonary function and exercise performances were assessed using resting heart rate (RHR), maximum heart rate (HRmax), heart rate recovery (HRR), and a 6-min walk test (6MWT) at the baseline and after 16 weeks of training.
Results: The MIS group demonstrated a significant reduction in RHR compared to the CON and MIL groups. While both exercise rehabilitation groups exhibited improvement in HRR, only the MIS group achieved a statistically significant improvement compared to the CON group. Post-training HRmax and 6MWT performance increased in both MIS and MIL groups, with only the MIL group presenting statistical significance compared to the CON group.
Conclusion: Exercise rehabilitation with different training regimens can enhance cardiac function in patients with CAD. Different heart rate modulation strategies yielded distinct effects on cardiopulmonary function. Maintenance of a narrower heart rate fluctuation during exercise was observed to significantly enhance the effectiveness of rehabilitation, which could lead to new treatment protocols or optimization of existing strategies for patients with cardiovascular conditions. The combination of 6MWT and power bicycle training may offer an effective method for improving cardiac function in community-based rehabilitation settings.
Keywords: Coronary artery disease; Exercise intensity; Aerobic exercise
| Introduction | ▴Top |
Coronary artery disease (CAD) is a common chronic disorder that contributes significantly to morbidity and mortality, imposing a substantial burden on the public health system [1]. While current pharmacological and surgical interventions can effectively alleviate coronary artery stenosis and restore myocardial perfusion [2], they fail to address the underlying pathophysiology of CAD. Abnormal resting heart rate (RHR) and heart rate recovery (HRR) are two established independent risk factors for the prediction of the onset and mortality of CAD. Specifically, an HRR of ≤ 12 beats/min indicates cardiac autonomic dysfunction with significant prognostic value in patients with coronary heart disease [3-6]. It is well-recognized that surgical interventions alone have limited efficacy in correcting RHR and HRR, and inadequate rehabilitation may lead to the recurrence of coronary artery stenosis in post-surgical patients [2]. Therefore, additional supportive treatments remain a clinically unmet need for the optimization and maintenance of long-term outcomes.
In recent years, exercise has emerged as a clinically validated intervention for the management of CAD. Studies have demonstrated that regular exercise training can mitigate cardiac ischemia, delay or eliminate angina pectoris, enhance endothelial function, and reduce the overall incidence of cardiovascular events [1]. Additionally, exercise has been associated with a 15% to 28% reduction in all-cause mortality in patients with heart diseases [7, 8]. Given this significant benefit, exercise intensity is a critical determinant of its therapeutic efficacy. Thus, it is critical to establish safe and effective aerobic exercise parameters when developing exercise prescriptions. However, despite the growing recognition of exercise as a core component of cardiac rehabilitation, there is currently no consensus regarding the specific effects of heart rate fluctuation variabilities on cardiopulmonary function [9, 10].
To provide a more reasonable and accessible exercise strategy for CAD management, this study was designed to evaluate the effects of different ranges of heart rate fluctuations during aerobic exercise using physiological parameters. Based on the preliminary results, we hypothesized that a smaller heart rate fluctuation during exercise would lead to greater improvements in cardiopulmonary functions.
| Materials and Methods | ▴Top |
Participants
Ninety-seven male patients diagnosed with stable CAD were enrolled between March 2017 and December 2019, all of whom had not yet met the criteria for surgical intervention. The inclusion criteria were: 1) meeting the European Society of Cardiology diagnostic criteria for stable CAD as outlined in the 2013 guidelines; 2) age between 40 and 70 years; 3) an education level of junior high school and above; and 4) no regular exercise habits. The exclusion criteria were: 1) aortic aneurysms; 2) acute large-area myocardial infarction; 3) acute pulmonary embolism; 4) new-onset or rapidly worsening confirmed angina; 5) multiple ventricular arrhythmia sources; and 6) progressive hemodynamic instability.
The study protocol was reviewed and approved by the hospital’s ethics committee (2020w10042), and the study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
The participants were randomly assigned to one of the three groups: the control (CON) group, the medium-intensity heart rate small range (MIS) group, and the medium-intensity heart rate large range (MIL) group. The experimental subjects were numbered from 1 to 97 using simple randomized grouping. Each number was divided by 3, and group allocation was determined based on the remainder: participants with no remainder were assigned to the CON group, those with a remainder of 1 were assigned to the MIL group, and those with a remainder of 2 were assigned to the MIS group. This process resulted in 31 participants in the CON group, 32 in the MIS group, and 34 in the MIL group. No significant differences in baseline characteristics were observed among the three groups, as shown in Table 1.
 Click to view | Table 1. Cohort Characteristics |
Evaluation indicators
Sports endurance index
Six-minute walk test (6MWT) [11] was conducted in a 50-m section of a corridor under controlled environmental conditions. A stool was available to accommodate the participants’ needs. Participants were instructed to walk back and forth at their maximal tolerable speed, and the distance walked within 6 min was recorded. Real-time heart rate (HR) was continuously recorded using a Polar heart rate monitor.
Heart function indicators
RHR [12] was recorded after the participant had remained at rest for 15 min. Maximum heart rate (HRmax) was recorded using a Polar heart rate monitor during the 6MWT. HRR [13] was defined as the reduction in heart rate 1 min after the completion of the exercise.
RHR and HRR were taken at the baseline and after the 16-week exercise intervention, while HRmax was assessed monthly. All assessments were performed by professional physiotherapists.
Personalized exercise prescription
The exercise regimen started with lower limb muscle resistance training for the first 4 weeks, followed by a personalized aerobic exercise regimen for the next 12 weeks. Muscle resistance training focused on isometric exercises targeting the quadriceps, hamstrings, gluteus medius, and adductors, performed three times per week. Each session consisted of three sets at an intensity of 15 repetitions maximum. For each exercise, the patient contracted the target muscle, maintained isometric contraction, lifted the lower limb off the bed, and held the position for 5 - 10 s. The 12-week aerobic exercise regimen involved recording the RHR and HRmax during the 6MWT to calculate the heart rate reserve (HRmax - RHR). The target heart rate range for moderate-intensity aerobic exercise was set at 40-60% of the heart rate reserve, plus the RHR [14], which was further refined for each group based on previous studies [2, 14] and clinical trials. The MIS group was assigned a heart rate range of RHR + 50% (HRmax - RHR) ± 2 bpm, while the MIL group had a range between RHR + 50% (HRmax - RHR) ± 6 bpm. The target heart rate range was adjusted monthly.
Power bicycle rehabilitation training
Power bicycles were utilized for structured rehabilitation training (Fig. 1). The seat height was adjusted to ensure the thighs were parallel to the ground when the pedals reached the highest position. Each training session began with quiet sitting for 5 min, followed by a 5-min warm-up without load applied. The initial cycling parameters were set at 15 W at a speed of 30 revolutions per minute (rpm), with an incremental increase of 5 rpm every minute to achieve the target heart rate range within 5 min. Real-time heart rate was monitored using the DASH 5000 system, with an on-screen heart rate displayed and an audio reminder every 5 s to instruct participants to maintain their target range. If participants experienced abnormal heart rate, palpitations, chest tightness, or shortness of breath, the training would be immediately halted, and appropriate measures would be administered under professional supervision.
 Click for large image | Figure 1. A 42-year-old male patient is exercising under the guidance of medical staff (model: SONE SERIAL#151-008739, SCIFIT Systems Inc.). |
Statistical analysis
All statistics were performed using IBM SPSS Statistics 26. Quantitative data were expressed as mean ± standard deviation (x ± s). For within-group comparisons of pre- and post-exercise regimens, the paired-sample t-test was used. For between-group comparisons post-exercise regimens, the Kruskal-walls test was applied. A P-value of less than 0.05 was considered statistically significant.
| Results | ▴Top |
General information
Four hundred and ninety patients were referred to cardiac rehabilitation, of which 140 met the inclusion and exclusion criteria. Forty patients withdrew and the remaining 100 patients went through randomization. Three patients discontinued for personal reasons (two from the CON group, and one from the MIS group). The baseline clinical characteristics of the participants were as follows: all 97 participants had one or two diseased vessels, 40 participants had a history of myocardial infarction, 44 participants had mild kidney abnormalities, and 31 participants had a family history of CAD. Regarding the medication in use, 68 participants were on beta-blockers, 61 were on anti-thrombotics, and 82 were on statins. No significant differences in patient characteristics were found among the CON, MIS, and MIL groups, as shown in Table 1.
MIS group performed best in cardiac function
After 16 weeks of rehabilitation training with specific regimens, the cardiopulmonary training results are summarized in Table 2. The MIS group showed a significant decrease in RHR compared to baseline (P < 0.01). Additionally, the post-training RHR in the MIS group was significantly lower than in both the CON and MIL groups (P < 0.01), while the MIL group showed no significant difference to the CON (P > 0.05). HRR increased significantly from pre- and post-training in both exercise groups (P < 0.01), while only the MIS group demonstrated a significant increase compared to the CON group (P < 0.01). No significant differences were observed in HRR between the MIS and MIL groups (P > 0.05).
 Click to view | Table 2. Changes in Cardiovascular Risk Parameters Throughout Training |
MIL group performed best in sports endurance
Regarding the HRmax, the MIL group showed a significant increase after training (P < 0.01), while the MIS group showed a modest increase (P < 0.05). Only the MIL group exhibited a significant increase in HRmax compared to the CON group (P < 0.01), with no significant difference observed between the CON and MIS groups. The 6MWT results followed a similar pattern, with the MIL group showing a significant increase compared to the CON group after training (P < 0.01), while no significant difference was found between the CON and MIS groups. However, significant improvements were observed between pre- and post-training within each exercise group (P < 0.01). No adverse events were reported during the training sessions.
Heart rate curve during exercise
Figure 2 presents the heart rate curve of two patients randomly selected from the two exercise groups during the exercise process after the first month of aerobic training. The data indicated that heart rate fluctuations in the MIL group were pronounced, as consistent heart rate oscillation was observed between the minimum and maximum recommended range throughout the exercise period. Similar fluctuation patterns could be observed in most MIL participants. In contrast, the MIS group showed heart rate variability within a more stable and narrow fluctuation range.
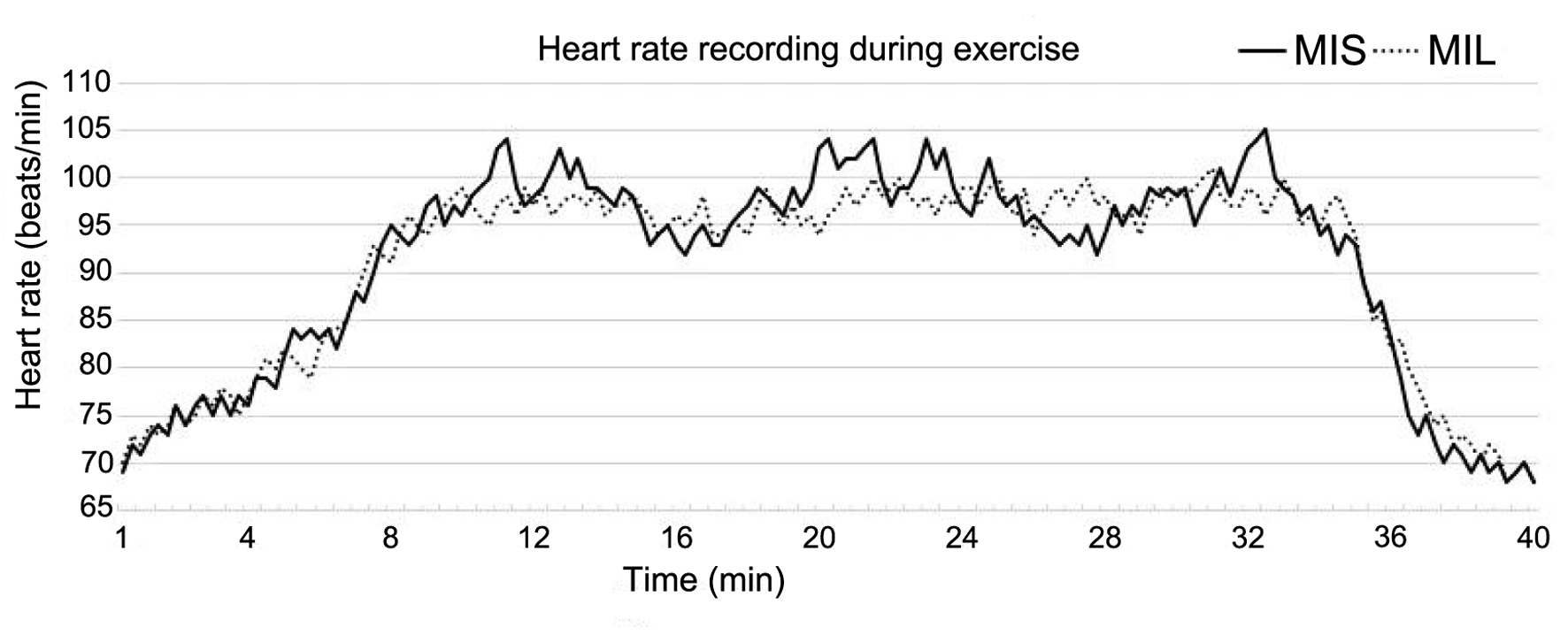 Click for large image | Figure 2. Heart rate change curve of two patients randomly selected from the two exercise groups during the exercise process after the first month of aerobic training. |
| Discussion | ▴Top |
In recent years, both physicians and healthcare researchers have increasingly recognized the importance of exercise therapy following cardiovascular surgery. Shreds of evidence have indicated that exercise therapy is effective across all stages of vascular disease management, significantly reducing adverse cardiovascular events and improving patient autonomic nerve function, exercise endurance, and quality of life [9, 10, 15-17]. Current aerobic exercise rehabilitation protocols largely follow the “ACSM Exercise Testing and Exercise Prescription Guidelines”, which recommend an exercise intensity of 40% to 60% of heart rate reserve for CAD [14]. This protocol has been widely used in clinical practices. However, our preclinical experiments based on the protocol [14] for CAD rehabilitation showed mixed results. Overall, the cardiopulmonary function of patients improved after exercise, supporting the efficacy of aerobic exercise as a beneficial intervention for patients with CAD [7, 15]. However, the degree of improvement varies among individuals, suggesting a need to reassess exercise details in certain cases. In this study, detailed experimental review and data analysis revealed that patients who closely monitored heart rate fluctuations during exercise tended to achieve better outcomes. This observation led to the investigation of whether maintaining a specific heart rate range or allowing wide fluctuation would yield differing results. Despite an extensive literature review, no clear consensus was found. With the hypothesis that smaller and more consistent heart rate fluctuations during exercise could better regulate sympathetic nerve activity, thus improving cardiac function, this study was designed to provide clarity and shed light on the standardization of rehabilitation regimens for future clinical practices. The 6MWT was conducted for maximum heart rate determination and to establish moderate-intensity exercise loads for the intervention groups. For the MIS group, the exercise heart rate range was defined as RHR plus 50% of heart rate reserve, with fluctuations of ± 2 bpm, while the MIL group had a fluctuation range of ± 6 bpm. This range corresponded to approximately 40% to 60% of heart rate reserve. Additionally, isometric resistance training was introduced 1 month before the study to reduce the risk of joint pain and muscle atrophy, which could compromise adherence.
Our findings showed significant improvements in cardiopulmonary function indicators after the exercise interventions. Both the MIS and MIL groups showed significant increases in the HRR, HRmax, and 6MWT performance compared to baseline, consistent with previous studies [9, 16]. Firstly, increased RHR has been shown to activate the sympathetic nervous system, increasing myocardial oxygen demand, and reducing cardiac reserve, thereby worsening myocardial ischemia [18]. Therefore, lowering RHR is crucial for patients with coronary heart disease. In this study, post-exercise RHR in the MIS group was significantly lower than that in both the CON and MIL groups. Secondly, HRR, another key indicator of cardiac autonomic nervous activity was analyzed, as it serves as a valuable prognostic marker for patient outcomes [9, 19-21]. The MIS group showed significantly improved HRR compared to the CON group, whereas no such difference was found in the MIL group. These combined results of RHR and HRR suggest that continuous and stable exercise stimuli may be more effective in reducing RHR when performing aerobic exercise. One of the potential explanations is that smaller heart rate fluctuations during exercise could lead to more stable autonomic regulation, which may result in improved cardiac function. Moreover, smaller fluctuations indicate lower strain on the cardiomyocyte, allowing for a more consistent workload on the heart, which may promote better long-term cardiac function. Future studies will further explore the optimal heart rate range and the underlying mechanisms for the optimization of cardiopulmonary outcomes from physical rehabilitation in patients with CAD. Lastly, HRmax and 6MWT performances were significantly improved in the MIL group compared to the CON group, suggesting that larger heart rate fluctuations during exercise may enhance coronary blood perfusion and improve peak exercise capacity. This could be particularly beneficial for patients with higher physical fitness levels, as it may enhance extreme exercise tolerance. However, no significant differences were observed between the two exercise groups, warranting further research for the development of personalization rehabilitation regimens in clinical practices.
In conclusion, this study highlighted the significance of heart rate fluctuation range during aerobic exercise on cardiopulmonary outcomes in CAD patients. Results have suggested that smaller heart rate fluctuations may provide greater benefits in RHR reduction and thus cardiac autonomic function enhancement. While larger fluctuations could lead to exercise endurance and peak capacity improvements. Lastly, in-depth analyses are planned to gather additional information, optimize medical resource allocation, and reduce the medical burden on patients. Considering the practicality of sports rehabilitation equipment and the feasibility of long-term monitoring and follow-up, the combination of 6MWT and power bicycle training may offer an effective strategy to enhance cardiac function in community rehabilitation settings.
Limitations
The primary limitations of this study include the relatively small sample size. Additionally, the exercise prescription used in the study may not represent the optimal protocol and requires refinement for future studies. The fact that all participants in this study were male further limits the generalizability of the results, particularly for patients with more severe or prolonged heart diseases. Future studies will aim to address these limitations by including a larger and more diverse population. Although the exercise tolerance measured by the 6MWT correlated well with the maximum exercise capacity obtained through cardiopulmonary exercise testing (CPET) [17], it may not fully capture exercise tolerance in patients with CAD [22]. To enhance the objectivity and accuracy of future studies, CPET will be incorporated for the maximum heart rate measurement and provide a more accurate assessment of exercise tolerance [23].
Acknowledgments
We express our gratitude to the patients, doctors, and nurses who contributed to the research and data collection.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author Contributions
Jun Hong Liu contributed to the conception or design of the study, as well as data acquisition, analysis, and interpretation. Hui Hui Song and Hua Fang Zhang contributed to drafting the manuscript. Jia Lin Ji revised the manuscript critically for important intellectual content. Xue Jiao Zhou and Xi Cai Sun contributed to the final approval of the version to be published.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
BMI: body mass index; CAD: coronary artery disease; HRmax: maximum heart rate; HRmax-RHR: maximum heart rate - resting heart rate; HRR: heart rate recovery; MIS: medium-intensity heart rate small range; MIL: medium-intensity heart rate large range; RHR: resting heart rate; 6MWT: six-minute walk test; SD: standard deviation
| References | ▴Top |
- Anderson L, Taylor RS. Cardiac rehabilitation for people with heart disease: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2014;2014(12):CD011273.
doi pubmed - Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165.
doi pubmed - Akyuz A, Alpsoy S, Akkoyun DC, Degirmenci H, Guler N. Heart rate recovery may predict the presence of coronary artery disease. Anadolu Kardiyol Derg. 2014;14(4):351-356.
doi pubmed - Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284(11):1392-1398.
doi pubmed - Maddox TM, Ross C, Ho PM, Masoudi FA, Magid D, Daugherty SL, Peterson P, et al. The prognostic importance of abnormal heart rate recovery and chronotropic response among exercise treadmill test patients. Am Heart J. 2008;156(4):736-744.
doi pubmed - Vivekananthan DP, Blackstone EH, Pothier CE, Lauer MS. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol. 2003;42(5):831-838.
doi pubmed - Vogiatzis I, Zakynthinos S. The physiological basis of rehabilitation in chronic heart and lung disease. J Appl Physiol (1985). 2013;115(1):16-21.
doi pubmed - Chrysohoou C, Angelis A, Tsitsinakis G, Spetsioti S, Nasis I, Tsiachris D, Rapakoulias P, et al. Cardiovascular effects of high-intensity interval aerobic training combined with strength exercise in patients with chronic heart failure. A randomized phase III clinical trial. Int J Cardiol. 2015;179:269-274.
doi pubmed - Kalka D, Domagala Z, Kowalewski P, Rusiecki L, Wojcieszczyk J, Koleda P, Marciniak W, et al. The influence of endurance training intensity on dynamics of post-exertional heart rate recovery adaptation in patients with ischemic heart disease. Adv Med Sci. 2013;58(1):50-57.
doi pubmed - Matsuo T, Saotome K, Seino S, Eto M, Shimojo N, Matsushita A, Iemitsu M, et al. Low-volume, high-intensity, aerobic interval exercise for sedentary adults: VO(2)max, cardiac mass, and heart rate recovery. Eur J Appl Physiol. 2014;114(9):1963-1972.
doi pubmed - Gomes E, Bastos T, Probst M, Ribeiro JC, Silva G, Corredeira R. Reliability and validity of 6MWT for outpatients with schizophrenia: A preliminary study. Psychiatry Res. 2016;237:37-42.
doi pubmed - Maciel BC, Gallo L, Jr., Marin Neto JA, Lima Filho EC, Martins LE. Autonomic nervous control of the heart rate during dynamic exercise in normal man. Clin Sci (Lond). 1986;71(4):457-460.
doi pubmed - Pumprla J, Howorka K, Groves D, Chester M, Nolan J. Functional assessment of heart rate variability: physiological basis and practical applications. Int J Cardiol. 2002;84(1):1-14.
doi pubmed - Pescatello LS. American College of Sports Medicine, eds. ACSM’s guidelines for exercise testing and prescription. 9th ed. Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014.
- Achttien RJ, Staal JB, van der Voort S, Kemps HM, Koers H, Jongert MW, Hendriks EJ, et al. Exercise-based cardiac rehabilitation in patients with coronary heart disease: a practice guideline. Neth Heart J. 2013;21(10):429-438.
doi pubmed - Jelinek HF, Huang ZQ, Khandoker AH, Chang D, Kiat H. Cardiac rehabilitation outcomes following a 6-week program of PCI and CABG Patients. Front Physiol. 2013;4:302.
doi pubmed - Deniz Acar R, Bulut M, Ergun S, Yesin M, Alici G, Akcakoyun M. Effect of cardiac rehabilitation on left atrial functions in patients with acute myocardial infarction. Ann Phys Rehabil Med. 2014;57(2):105-113.
doi pubmed - Sarlon J, Habich O, Schneider B. Elevated rest heart rate in psychiatric patients and different effects of psychotropic medication. Pharmacopsychiatry. 2016;49(1):18-22.
doi pubmed - Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol. 2004;44(2):423-430.
doi pubmed - Arena R, Guazzi M, Myers J, Peberdy MA. Prognostic value of heart rate recovery in patients with heart failure. Am Heart J. 2006;151(4):851.e857-813.
doi pubmed - Bond V, Mills RM, Caprarola M, Vaccaro P, Adams RG, Blakely R, Roltsch M, et al. Aerobic exercise attenuates blood pressure reactivity to cold pressor test in normotensive, young adult African-American women. Ethn Dis. 1999;9(1):104-110.
pubmed - Cahalin LP, Arena R, Labate V, Bandera F, Lavie CJ, Guazzi M. Heart rate recovery after the 6 min walk test rather than distance ambulated is a powerful prognostic indicator in heart failure with reduced and preserved ejection fraction: a comparison with cardiopulmonary exercise testing. Eur J Heart Fail. 2013;15(5):519-527.
doi pubmed - Milani RV, Lavie CJ, Mehra MR, Ventura HO. Understanding the basics of cardiopulmonary exercise testing. Mayo Clin Proc. 2006;81(12):1603-1611.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.