| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 2, April 2025, pages 110-119
A Clinical Retrospective Study on the Combined Use of Monocyte-to-Lymphocyte Ratio and Triglyceride-Glucose Index to Predict the Severity of Coronary Artery Disease
Bin Gua, Dan Lib, Min Lib, Kaisen Huanga, c
aDepartment of Cardiology, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan, China
bDepartment of Cardiology, Neijiang Dongxing District People’s Hospital, Neijiang 641300, Sichuan, China
cCorresponding Author: Kaisen Huang, Department of Cardiology, Affiliated Hospital of Southwest Medical University, Luzhou, 646000, Sichuan, China
Manuscript submitted November 9, 2024, accepted February 7, 2025, published online February 18, 2025
Short title: MLR and TyG Index as Predictors of CAD Severity
doi: https://doi.org/10.14740/cr2006
| Abstract | ▴Top |
Background: Coronary artery disease (CAD) remains a leading cause of morbidity and mortality. Traditional risk models based on factors like age, hypertension, and lipid levels are limited in individualized prediction, especially for high-risk populations. This study evaluates the independent and combined predictive value of the monocyte-to-lymphocyte ratio (MLR) and triglyceride-glucose (TyG) index for assessing CAD severity.
Methods: In this single-center, retrospective study, 678 patients who underwent coronary angiography (CAG) between January 2022 and June 2024 were included. Eligible patients were aged ≥ 40 years with suspected or confirmed CAD. Clinical data and laboratory values were extracted from electronic records. MLR was calculated as the monocyte-to-lymphocyte ratio, and TyG index was derived from fasting triglycerides and glucose. CAD severity was categorized by SYNTAX scores into no CAD, mild, moderate, and severe CAD. Statistical analyses included Spearman correlation, multivariate logistic regression, and receiver operating characteristic (ROC) curve analysis to assess the diagnostic accuracy of MLR and TyG index.
Results: Of the 678 patients, 67.1% had CAD. Both MLR and TyG index were significantly associated with CAD severity, with MLR showing a stronger correlation with SYNTAX scores. Multivariate analysis confirmed MLR (odds ratio (OR) = 2.15) and TyG index (OR = 1.75) as independent predictors of CAD. The combined MLR-TyG model achieved an area under the curve (AUC) of 0.804, surpassing the predictive value of each marker alone. Subgroup analysis indicated high predictive accuracy in diabetic and hypertensive patients.
Conclusions: MLR and TyG index independently and jointly predict CAD severity, with the combined model enhancing diagnostic accuracy. Reflecting both inflammatory and metabolic dysfunction, this dual-marker approach offers a practical tool for CAD risk stratification, particularly in high-risk populations. Further multicenter studies are needed to validate these findings and examine additional biomarker combinations to refine CAD risk models.
Keywords: Monocyte-to-lymphocyte ratio; Triglyceride-glucose index; Coronary artery disease; Risk prediction; Atherosclerosis
| Introduction | ▴Top |
Coronary artery disease (CAD) is a leading cause of death and disability worldwide [1, 2]. The primary mechanism is atherosclerosis, a chronic inflammatory process driven by lipid accumulation, endothelial dysfunction, and immune responses, which narrows coronary arteries and increases the risk of myocardial infarction and sudden death [3, 4]. Early detection and accurate risk assessment are essential to reducing CAD-related complications [5].
Traditional CAD risk models, including the Framingham risk score and SCORE, rely on factors like age, gender, smoking, hypertension, and lipid levels [6, 7]. While valuable for population-level assessment, these models often fall short in personalized prediction, especially among high-risk individuals, such as those with diabetes or metabolic syndrome [8]. This limitation has spurred interest in novel biomarkers that can improve early identification and precise risk stratification. Recently, the monocyte-to-lymphocyte ratio (MLR) and triglyceride-glucose (TyG) index have shown promise in predicting CAD and its severity [9, 10].
MLR reflects the balance between pro-inflammatory monocytes and regulatory lymphocytes, key players in atherosclerosis. Monocytes promote plaque formation and destabilization, while lymphocytes modulate immune responses with anti-inflammatory effects [11, 12]. MLR has demonstrated superior predictive value for CAD severity compared to other inflammatory indices like the neutrophil-to-lymphocyte ratio [13, 14].
The TyG index, derived from fasting triglycerides and glucose, serves as a surrogate marker for insulin resistance [15] and reflects broader metabolic dysfunction by integrating lipid abnormalities and insulin resistance [16]. Recent evidence has further established its value as a promising diagnostic biomarker across various diseases [17]. This comprehensive metabolic indicator has shown strong predictive value for cardiovascular outcomes, especially in individuals with metabolic disturbances [18, 19].
This study is one of the few to comprehensively evaluate the combined predictive value of MLR and TyG index for CAD severity. While previous studies have explored the individual or combined effects of these markers [20], our research expands this scope by investigating their predictive performance across subgroups, including gender, diabetes, and hypertension, to assess their robustness and clinical applicability in diverse populations. We hypothesize that their joint use will significantly improve risk prediction accuracy [21]. By examining their relationships with the SYNTAX score, a measure of coronary artery complexity [22], we aim to provide evidence for incorporating these biomarkers into clinical CAD risk assessment. This approach offers clinicians a practical tool for individualized risk stratification, particularly for patients with inflammatory and metabolic dysfunction.
| Materials and Methods | ▴Top |
Study design and population
This single-center, retrospective cohort study was conducted at Neijiang Dongxing District People’s Hospital from January 2022 to June 2024. The institutional ethics committee approved the study protocol, and informed consent was waived due to the retrospective design. This study was conducted in compliance with the ethical standards of the responsible institution for research on human subjects, adhering to the principles outlined in the Declaration of Helsinki. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [23].
We enrolled 678 consecutive patients who underwent coronary angiography (CAG). Inclusion criteria were: 1) age ≥ 40 years, and 2) CAG performed for suspected or confirmed CAD. Exclusion criteria included: 1) prior coronary revascularization; 2) acute infection, autoimmune disease, or malignancy; 3) severe hepatic insufficiency (Child-Pugh class C) or renal insufficiency (estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2); 4) significant valvular heart disease or cardiomyopathy; and 5) pregnancy.
Clinical data collection
Demographic, clinical, and laboratory data were systematically extracted from electronic medical records using standardized forms. Collected variables included age, gender, smoking status, medical history (e.g., hypertension, diabetes), body mass index (BMI), blood pressure, and laboratory parameters. Fasting blood samples were collected and analyzed in the hospital’s central laboratory following standard protocols.
MLR was calculated as the absolute monocyte count divided by the absolute lymphocyte count. The TyG index was calculated using the formula: TyG index = ln(TG (mg/dL) × FBG (mg/dL)/2), with triglyceride (TG) and fasting blood glucose (FBG) values converted from mmol/L to mg/dL [9].
Coronary angiography and SYNTAX score
CAD was defined as ≥ 50% diameter stenosis in any major epicardial coronary artery, based on current guidelines [6]. All CAG procedures adhered to standardized protocols, and two experienced interventional cardiologists (each with > 10 years of experience) independently reviewed the angiograms. Discrepancies were resolved by consensus.
The SYNTAX score, calculated using an online tool [24], quantified the complexity of coronary artery lesions according to established criteria [22]. Patients were stratified into four categories based on SYNTAX scores: no CAD (0 points), mild CAD (1 - 22 points), moderate CAD (23 - 32 points), and severe CAD (≥ 33 points).
Statistical analysis
Statistical analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). Sample size calculations were based on previous studies, with an estimated area under the curve (AUC) difference of 0.1 between combined and individual markers (α = 0.05, β = 0.2). Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range) based on normality testing (Kolmogorov-Smirnov test), while categorical variables were presented as counts and percentages.
Between-group comparisons were conducted using independent t-tests or Mann-Whitney U tests for continuous variables and Chi-square tests for categorical variables. Spearman correlation coefficients were used to evaluate relationships between MLR, TyG index, and SYNTAX scores. Multivariate logistic regression analysis, adjusting for established risk factors, assessed the independent predictive value of MLR and TyG index. Receiver operating characteristic (ROC) curves were used to evaluate diagnostic performance, with AUCs compared using DeLong’s test. Predefined subgroup analyses were conducted to examine the predictive value of MLR and TyG index in various populations. A two-sided P-value < 0.05 was considered statistically significant.
| Results | ▴Top |
Baseline characteristics
The study included 678 patients, with 453 (67.1%) diagnosed with CAD and 225 (32.9%) showing no CAD. The average age was 60.9 ± 9.85 years, and 46.5% were male. Based on SYNTAX scores, patients were classified into four severity categories: no CAD (32.9%), mild CAD (25.1%), moderate CAD (22.7%), and severe CAD (19.3%).
Compared to the non-CAD group, patients in the CAD group were older (63.33 ± 9.32 vs. 55.94 ± 9.03 years, P < 0.01) and had a higher proportion of males (53.8% vs. 31.4%, P < 0.01). Comorbidities such as hypertension (75.4% vs. 48.9%, P < 0.01) and diabetes (32.1% vs. 12.1%, P < 0.01) were more prevalent in the CAD group. Both MLR (2.40 (1.72 - 3.08) vs. 1.84 (1.32 - 2.36), P < 0.01) and TyG index (4.89 (4.57 - 5.21) vs. 4.63 (4.32 - 4.94), P < 0.01) were significantly elevated in CAD patients, indicating an association between these markers and CAD presence (Table 1). Based on the study population, preliminary reference ranges for MLR and TyG index can be proposed: 1.84 - 2.40 for MLR and 4.63 - 4.89 for TyG index in non-CAD patients, and 2.40 - 3.08 for MLR and 4.89 - 5.21 for TyG index in CAD patients. These ranges suggest potential clinical cut-offs; however, larger studies are necessary to validate these thresholds and explore their generalizability.
 Click to view | Table 1. Baseline Characteristics of the Study Population |
Association between MLR, TyG index, and CAD
Spearman correlation analysis showed a significant positive correlation between MLR and CAD presence (r = 0.446, P <0.001) as well as between TyG index and CAD (r = 0.361, P < 0.001). Additionally, MLR had a stronger correlation with SYNTAX scores (r = 0.578, P < 0.001) compared to TyG index (r = 0.515, P < 0.001), suggesting that both markers are associated with CAD severity (Table 2).
 Click to view | Table 2. Spearman Correlation Analysis of MLR and TyG Index With CAD and Its Severity |
Multivariate logistic regression analysis, adjusted for age, gender, smoking status, hypertension, diabetes, BMI, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), confirmed that both MLR (odds ratio (OR) = 2.15, 95% confidence interval (CI): 1.40-3.28, P < 0.001) and TyG index (OR = 1.75, 95% CI: 1.30 - 2.36, P < 0.001) were independent predictors of CAD (Table 3).
 Click to view | Table 3. Multivariate Logistic Regression Analysis of Factors Associated With CAD |
Diagnostic performance of MLR and TyG index
ROC curve analysis evaluated the diagnostic performance of MLR and TyG index for predicting CAD. MLR achieved an AUC of 0.774 (95% CI: 0.739 - 0.809, P < 0.001) with an optimal cut-off of 1.77, yielding a sensitivity of 70.1% and specificity of 75.8%. The TyG index showed an AUC of 0.722 (95% CI: 0.682 - 0.762, P < 0.001) with an optimal cut-off of 4.71, resulting in a sensitivity of 78.2% and specificity of 68.4%. The combined model of MLR and TyG index improved predictive performance, reaching an AUC of 0.804 (95% CI: 0.771 - 0.837, P < 0.001), with an optimal cut-off of 6.50, sensitivity of 73.8%, and specificity of 74.9% (Fig. 1). Additionally, the predictive performance of the combined MLR and TyG index model (AUC = 0.804) was compared to the Framingham risk model, which achieved an AUC of 0.784 (95% CI: 0.750 - 0.818). The combined model demonstrated superior diagnostic accuracy, emphasizing the value of integrating inflammatory and metabolic markers.
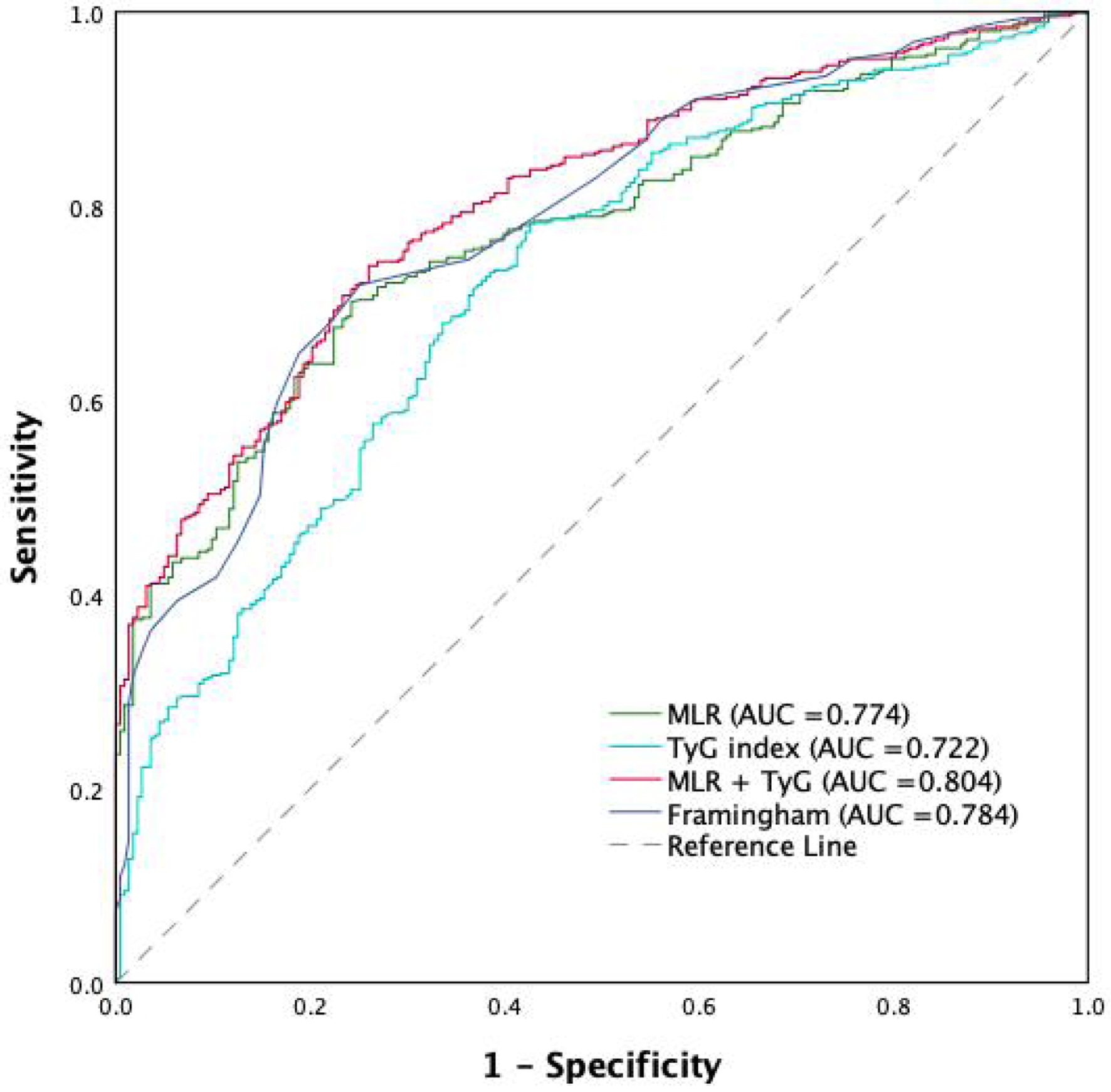 Click for large image | Figure 1. ROC curve comparing the predictive performance of MLR, TyG index, their combination (MLR and TyG index), and the Framingham risk model for CAD severity. CAD: coronary artery disease; MLR: monocyte-to-lymphocyte ratio; ROC: receiver operating characteristic; TyG: triglyceride-glucose. |
Association between MLR, TyG index, and CAD severity
The Kruskal-Wallis test revealed significant differences in MLR across CAD severity groups (χ2(3) = 267.462, P < 0.001), with MLR levels increasing with CAD severity. Similarly, significant differences were observed in TyG index among severity groups (χ2(3) = 116.466, P < 0.001). The combined use of MLR and TyG index yielded the most pronounced differences across severity levels (χ2(3) = 289.755, P < 0.001), suggesting that the combined model provides a better distinction among varying degrees of CAD severity (Fig. 2).
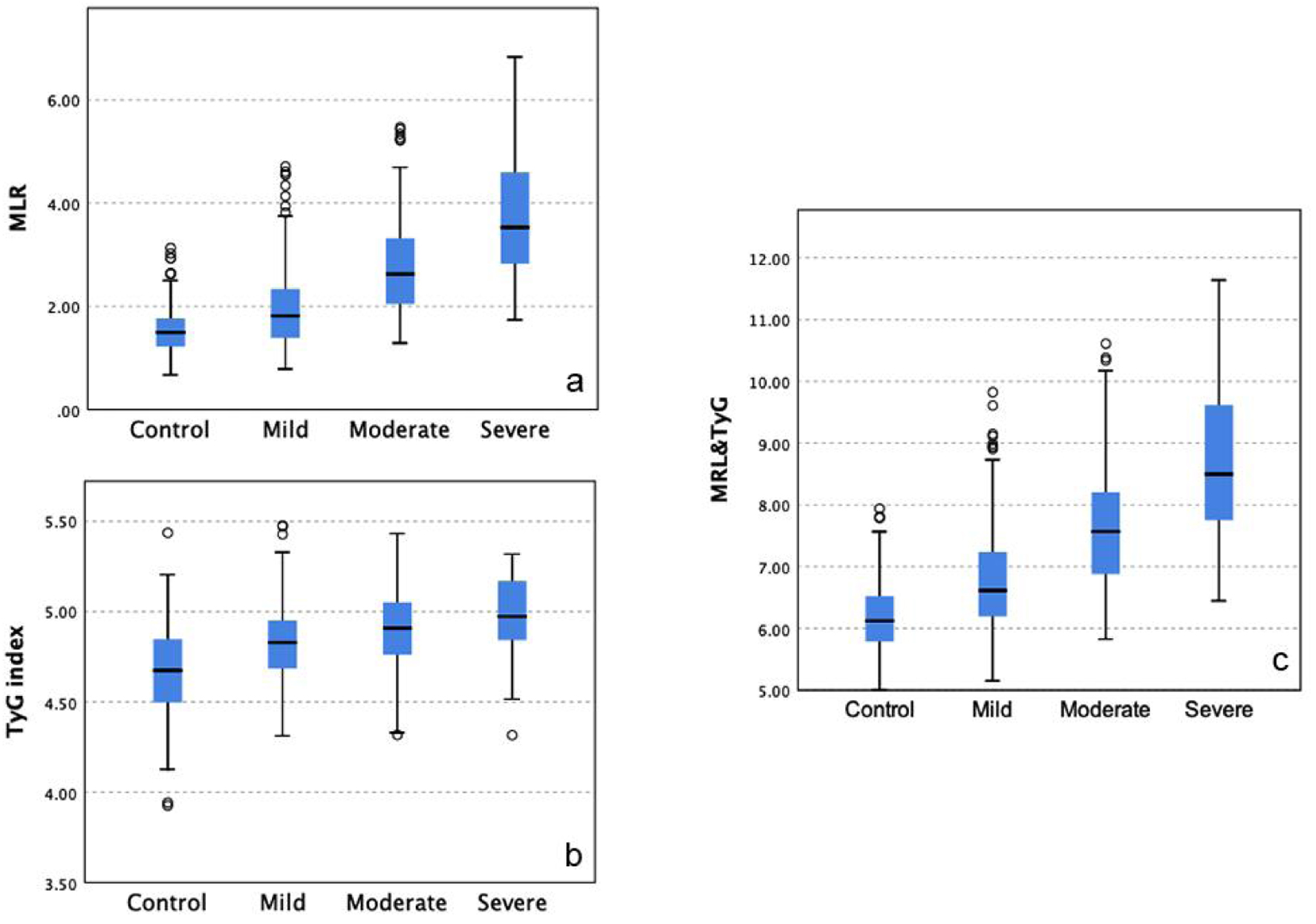 Click for large image | Figure 2. Differences in MLR and TyG index across CAD severity groups. Significant differences were observed in MLR (a) and TyG index (b) across CAD severity groups, with the combined model (c) showing the most pronounced distinction. CAD: coronary artery disease; MLR: monocyte-to-lymphocyte ratio; TyG: triglyceride-glucose. |
Multivariate logistic regression analysis further indicated that MLR and TyG index remained independent predictors of CAD severity. Each unit increase in MLR was associated with a 0.90-fold (95% CI: 0.53 - 1.28, P < 0.001), 1.93-fold (95% CI: 1.47 - 2.38, P < 0.001), and 2.68-fold (95% CI: 2.18 - 3.18, P < 0.001) increased risk of mild, moderate, and severe CAD, respectively. Similarly, each unit increase in TyG index corresponded to a 2.13-fold (95% CI: 1.18 - 3.09, P < 0.001), 3.03-fold (95% CI: 1.66 - 4.39, P < 0.001), and 4.30-fold (95% CI: 2.64 - 5.74, P < 0.001) increased risk for mild, moderate, and severe CAD, respectively (Table 4).
 Click to view | Table 4. Association of MLR and TyG Index With CAD Severity |
Subgroup analysis
Subgroup analysis revealed that the predictive performance of the MLR and TyG index model differed between men and women. The combined model achieved an AUC of 0.85 (95% CI: 0.81 - 0.89) in men, significantly higher than in women (AUC = 0.82, 95% CI: 0.75 - 0.88, P < 0.05). In contrast, the sensitivity and specificity of the model showed no statistically significant differences between men (75.2% and 73.5%, respectively) and women (71.8% and 72.9%, respectively; P > 0.05). These findings suggest that sex-specific factors may influence the model’s AUC performance, but their impact on sensitivity and specificity requires further investigation.
In diabetic patients, the combined model achieved an AUC of 0.88 (95% CI: 0.83 - 0.92), significantly higher than in non-diabetic patients (AUC = 0.81, 95% CI: 0.75 - 0.87, P < 0.05). Sensitivity and specificity were 79.3% and 76.1% in diabetic patients, compared to 72.8% and 70.5% in non-diabetic patients, respectively. Similarly, hypertensive patients showed better model performance with an AUC of 0.84 (95% CI: 0.79 - 0.89) compared to non-hypertensive patients (AUC = 0.80, 95% CI: 0.73 - 0.85, P < 0.05). These results emphasize the strong predictive value of the MLR and TyG index model in high-risk populations such as those with diabetes and hypertension (Fig. 3).
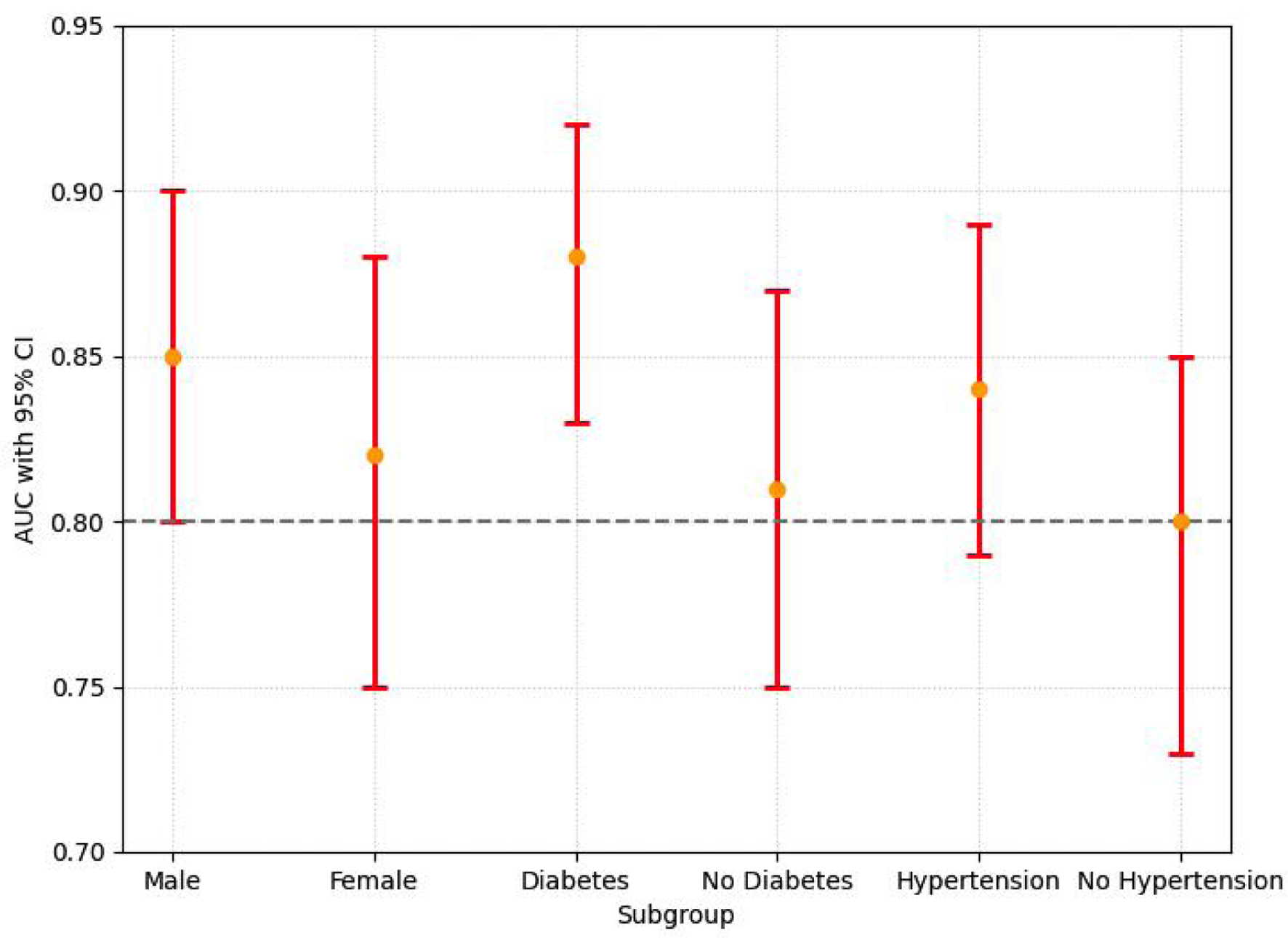 Click for large image | Figure 3. The AUC values with 95% CI for subgroups (male, female, diabetes, no diabetes, hypertension, and no hypertension) are shown. Significant differences in AUC were observed between men and women (P < 0.05), and between diabetic and non-diabetic patients (P < 0.05). No significant differences in sensitivity and specificity were found between subgroups (not shown). Orange dots represent AUC values, and red bars represent the 95% CI. AUC: area under the curve; CI: confidence interval. |
| Discussion | ▴Top |
This study demonstrates that both MLR and TyG index independently predict CAD severity, with their combination achieving superior diagnostic accuracy (AUC = 0.804). These findings underscore the complementary roles of inflammation and metabolic dysfunction in CAD pathophysiology [4, 11]. The interaction between immune and metabolic pathways in atherosclerosis reflects the integrated nature of CAD progression [25].
MLR has emerged as a valuable inflammatory biomarker, balancing pro-inflammatory monocytes and regulatory lymphocytes. Our results show a strong association between MLR and CAD severity (OR = 2.68 for severe CAD), consistent with evidence supporting its role in cardiovascular risk stratification and mortality prediction [10, 26, 27]. The stronger correlation of MLR with SYNTAX scores (r = 0.578) compared to TyG index suggests that inflammation plays a crucial role in complex coronary lesions [13]. Additionally, studies indicate that combining MLR with other inflammatory markers enhances CAD risk prediction [28].
The TyG index, a marker of insulin resistance, also showed significant predictive value for CAD severity (OR = 4.30 for severe CAD), aligning with recent meta-analyses linking TyG index to adverse cardiovascular events [9, 18]. As a validated marker in cardiovascular risk assessment [29], TyG index has gained increasing recognition. A recent dose-response meta-analysis further established a robust link between TyG index levels and cardiovascular outcomes, with each unit increase in TyG index associated with proportional increases in risk [30]. This association reflects the impact of metabolic dysfunction on atherosclerosis progression, positioning TyG index as a key marker for CAD risk, particularly in patients with metabolic disorders [16]. Notably, longitudinal studies confirm TyG index’s predictive value for cardiovascular events in both diabetic and non-diabetic populations [31].
Although the combined model achieved an AUC of 0.804, its sensitivity (73.8%) remains below the ideal threshold for clinical screening tools, which typically aim for sensitivity levels above 90%. This limitation suggests the need for further optimization by incorporating additional biomarkers or utilizing longitudinal data to enhance diagnostic accuracy. This approach is practical - given the availability and cost-effectiveness of the measurements - and offers complementary pathophysiological insights [21]. Evidence from multi-marker scoring systems supports this combined approach, showing improved accuracy by reflecting different pathological mechanisms [32]. Compared to the Framingham risk model (AUC = 0.784), the combined MLR and TyG index model (AUC = 0.804) offered better predictive accuracy. This suggests that while traditional risk models are valuable, their reliance on demographic and classical risk factors may not fully capture the inflammatory and metabolic processes underlying CAD. Our subgroup analysis showed particularly strong predictive value in diabetic (AUC = 0.88) and hypertensive patients (AUC = 0.84), further supporting its utility in high-risk populations [8].
The observed differences in AUC between men and women highlight the need to address sex-specific disparities in CAD risk prediction. Women frequently exhibit pathophysiological features such as non-obstructive CAD, microvascular dysfunction, and diffuse plaque morphology - phenotypes poorly captured by anatomical scoring systems like SYNTAX scores [33, 34]. These features are often driven by systemic inflammation and insulin resistance, which may explain why the MLR and TyG index model, despite its lower AUC in women (0.82 vs. 0.85 in men), maintains comparable sensitivity and specificity across sexes. Specifically, MLR reflects monocyte-driven plaque instability (OR = 2.68 for severe CAD), while TyG index (OR = 4.30) captures metabolic dysfunction linked to endothelial oxidative stress [16]. Their combined use may bypass the limitations of anatomical scoring by targeting upstream inflammatory-metabolic pathways, offering a critical advantage for women with atypical CAD presentations [34, 35].
This study has several limitations. First, as a single-center retrospective study, selection bias and limited generalizability are inherent. Expanding to multicenter designs with more diverse populations would enhance the robustness and applicability of the findings [1]. Second, single time-point measurements do not capture the dynamic changes of biomarkers, limiting the understanding of temporal variations in CAD progression [36]. Third, BMI was used as a surrogate measure for obesity, which may inadequately reflect metabolic risks. Alternative metrics, such as waist-to-hip ratio or body fat percentage, should be explored to better evaluate CAD risk in future studies [37]. Fourth, the SYNTAX II score, while widely used, may underestimate CAD severity in women due to its focus on anatomical complexity rather than microvascular dysfunction - a hallmark of female-pattern CAD [34, 36]. Future studies should validate sex-adapted scoring systems that integrate both anatomical and biomarker-driven parameters [34, 35]. Fifth, the narrow range of MLR and TyG index values highlights the need for standardized reference thresholds to improve clinical utility. While this study provides preliminary reference ranges, validation in larger, more diverse cohorts is essential to establish actionable cut-offs. Sixth, this study did not consider social determinants of health (SDOH), such as socioeconomic status and access to care, which significantly influence cardiovascular health [38]. Future studies should incorporate SDOH for a more comprehensive CAD risk assessment. Finally, the absence of high-sensitivity C-reactive protein (hs-CRP), a well-established inflammatory biomarker associated with CAD risk, limits the study’s ability to fully evaluate the inflammatory component of CAD. This limitation stems from the unavailability of hs-CRP data in the retrospective dataset. Future research should integrate hs-CRP with MLR and TyG index to enhance sensitivity and specificity, as combining inflammatory and metabolic markers could provide a more comprehensive risk stratification framework [39].
Future research should prioritize the following areas: 1) validating these findings in multicenter prospective studies to improve generalizability, 2) exploring biomarker dynamics over time to assess temporal changes in CAD progression [31], 3) integrating MLR and TyG index with emerging biomarkers like hs-CRP to develop more comprehensive risk stratification models, 4) conducting implementation studies to evaluate the clinical utility of this model in real-world settings, 5) investigating the cost-effectiveness of incorporating this model into existing healthcare systems, and 6) developing sex-stratified risk models by incorporating hormonal and microvascular biomarkers to optimize AUC performance in women. These steps will bridge the gap between research and clinical practice, ultimately improving CAD outcomes.
Conclusion
The combined MLR and TyG index model demonstrates significant potential as a practical, cost-effective tool for predicting CAD severity. By integrating inflammatory and metabolic markers, the model offers superior predictive performance compared to traditional risk models, particularly in high-risk populations such as diabetic and hypertensive patients. Its application in early screening and risk stratification can facilitate timely interventions and improve patient outcomes.
While the findings are promising, the single-center retrospective design limits generalizability. Future research should focus on validating these results through multicenter prospective studies and integrating the MLR and TyG index model into artificial intelligence (AI)-based frameworks. These advancements could enable the development of multi-biomarker risk prediction systems, addressing sex-specific and metabolic disparities while surpassing traditional models in both accuracy and clinical utility.
Acknowledgments
The authors would like to thank Southwest Medical University and Neijiang Dongxing District People’s Hospital for their strong support in this clinical retrospective study. Special thanks to the Department of Cardiology at Neijiang Dongxing District People’s Hospital for their assistance with data collection and case management. We are also grateful to all the patients who participated in this study; their involvement was essential to our analysis of biomarkers for coronary artery disease severity.
Financial Disclosure
No funding was received for this study.
Conflict of Interest
None to declare.
Informed Consent
Verbal informed consent was obtained and written informed consent was waived by the Ethical Committee of Neijiang Dongxing District People’s Hospital. This research was retrospective and consisted of surveys with reports presented no more than minimal risk of harm to subjects and involved no procedures for which written consent is normally required outside the research context.
Author Contributions
Bin Gu conducted the investigation, performed formal analysis, and prepared the original draft. Dan Li and Min Li contributed to the investigation. Kaisen Huang supervised the study, contributed to conceptualization and investigation, and reviewed and edited the manuscript.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
AUC: area under the curve; BMI: body mass index; CAD: coronary artery disease; CAG: coronary angiography; CI: confidence interval; eGFR: estimated glomerular filtration rate; FBG: fasting blood glucose; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; MLR: monocyte-to-lymphocyte ratio; NLR: neutrophil-to-lymphocyte ratio; OR: odds ratio; ROC: receiver operating characteristic; SD: standard deviation; STROBE: Strengthening the Reporting of Observational Studies in Epidemiology; SYNTAX: Synergy Between PCI With Taxus and Cardiac Surgery score; TG: triglycerides; TyG: triglyceride-glucose
| References | ▴Top |
- Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982-3021.
doi pubmed - Martin SS, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, et al. 2024 heart disease and stroke statistics: a report of US and global data from the American Heart Association. Circulation. 2024;149(8):e347-e913.
doi pubmed - Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115-126.
doi pubmed - Madaudo C, Coppola G, Parlati ALM, Corrado E. Discovering inflammation in atherosclerosis: insights from pathogenic pathways to clinical practice. Int J Mol Sci. 2024;25(11):6016.
doi pubmed - Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315-2381.
doi pubmed - Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(3):e18-e114.
doi pubmed - Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, Benetos A, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42(34):3227-3337.
doi pubmed - Hostacna L, Maslankova J, Pella D, Hubkova B, Marekova M, Pella D. A multi-biomarker approach to increase the accuracy of diagnosis and management of coronary artery disease. J Cardiovasc Dev Dis. 2024;11(9):258.
doi pubmed - Liang S, Wang C, Zhang J, Liu Z, Bai Y, Chen Z, Huang H, et al. Triglyceride-glucose index and coronary artery disease: a systematic review and meta-analysis of risk, severity, and prognosis. Cardiovasc Diabetol. 2023;22(1):170.
doi pubmed - Vakhshoori M, Nemati S, Sabouhi S, Shakarami M, Yavari B, Emami SA, Bondariyan N, et al. Prognostic impact of monocyte-to-lymphocyte ratio in coronary heart disease: a systematic review and meta-analysis. J Int Med Res. 2023;51(10):3000605231204469.
doi pubmed - Popa-Fotea NM, Ferdoschi CE, Micheu MM. Molecular and cellular mechanisms of inflammation in atherosclerosis. Front Cardiovasc Med. 2023;10:1200341.
doi pubmed - Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on A. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129-2138.
doi pubmed - Tudurachi BS, Anghel L, Tudurachi A, Sascau RA, Statescu C. Assessment of inflammatory hematological ratios (NLR, PLR, MLR, LMR and Monocyte/HDL-Cholesterol Ratio) in acute myocardial infarction and particularities in young patients. Int J Mol Sci. 2023;24(18):14378.
doi pubmed - Ji H, Li Y, Fan Z, Zuo B, Jian X, Li L, Liu T. Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: a syntax score assessment. BMC Cardiovasc Disord. 2017;17(1):90.
doi pubmed - Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK, Lee JH, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS One. 2014;9(2):e90430.
doi pubmed - Adams-Huet B, Jialal I. An increasing triglyceride-glucose index is associated with a pro-inflammatory and pro-oxidant phenotype. J Clin Med. 2024;13(13):3941.
doi pubmed - Sun Y, Ji H, Sun W, An X, Lian F. Triglyceride glucose (TyG) index: A promising biomarker for diagnosis and treatment of different diseases. Eur J Intern Med. 2025;131:3-14.
doi pubmed - Dang K, Wang X, Hu J, Zhang Y, Cheng L, Qi X, Liu L, et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003-2018. Cardiovasc Diabetol. 2024;23(1):8.
doi pubmed - Akbar MR, Pranata R, Wibowo A, Irvan, Sihite TA, Martha JW. The association between triglyceride-glucose index and major adverse cardiovascular events in patients with acute coronary syndrome - dose-response meta-analysis. Nutr Metab Cardiovasc Dis. 2021;31(11):3024-3030.
doi pubmed - Luo JW, Duan WH, Yu YQ, Song L, Shi DZ. Prognostic significance of triglyceride-glucose index for adverse cardiovascular events in patients with coronary artery disease: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:774781.
doi pubmed - Wang L, Wang Y, Wang W, Wang Z. Predictive value of triglyceride glucose index combined with neutrophil-to-lymphocyte ratio for major adverse cardiac events after PCI for acute ST-segment elevation myocardial infarction. Sci Rep. 2024;14(1):12634.
doi pubmed - Head SJ, Farooq V, Serruys PW, Kappetein AP. The SYNTAX score and its clinical implications. Heart. 2014;100(2):169-177.
doi pubmed - Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1-73.
doi pubmed - www.syntaxscore.com.
- Karadeni ZM, Aydin C, Demi Rkiran A, Alp C. Predictors of severity of coronary artery disease in patients with acute ST-elevation myocardial infarction. Namik Kemal Tip Dergisi. 2024:171-175.
- Hua Y, Sun JY, Lou YX, Sun W, Kong XQ. Monocyte-to-lymphocyte ratio predicts mortality and cardiovascular mortality in the general population. Int J Cardiol. 2023;379:118-126.
doi pubmed - de Liyis BG, Ciaves AF, Intizam MH, Jusuf PJ, Artha I. Hematological biomarkers of troponin, neutrophil-to-lymphocyte ratio, and monocyte-to-lymphocyte ratio serve as effective predictive indicators of high-risk mortality in acute coronary syndrome. Biomedicine (Taipei). 2023;13(4):32-43.
doi pubmed - Mohanty V, Sharma S, Goswami S, Kaushik A, Choudhary R, Yadav D, Deora S, et al. Association of novel hematological indices with severity of coronary artery disease using SYNTAX score in patients with acute coronary syndrome. Cardiovasc Hematol Disord Drug Targets. 2023;23(3):202-211.
doi pubmed - Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68.
doi pubmed - Wan Y, Zhang Z, Ling Y, Cui H, Tao Z, Pei J, Maimaiti A, et al. Association of triglyceride-glucose index with cardiovascular disease among a general population: a prospective cohort study. Diabetol Metab Syndr. 2023;15(1):204.
doi pubmed - Rafiee H, Mohammadifard N, Nouri F, Alavi Tabatabaei G, Najafian J, Sadeghi M, Boshtam M, et al. Association of triglyceride glucose index with cardiovascular events: insights from the Isfahan Cohort Study (ICS). Eur J Med Res. 2024;29(1):135.
doi pubmed - Sun C, Hu L, Li X, Zhang X, Chen J, Li D, Zhang J, et al. Triglyceride-glucose index's link to cardiovascular outcomes post-percutaneous coronary intervention in China: a meta-analysis. ESC Heart Fail. 2024;11(3):1317-1328.
doi pubmed - Lansky A, Baron SJ, Grines CL, Tremmel JA, Al-Lamee R, Angiolillo DJ, Chieffo A, et al. SCAI expert consensus statement on sex-specific considerations in myocardial revascularization. J Soc Cardiovasc Angiogr Interv. 2022;1(2):100016.
doi pubmed - Rajendran A, Minhas AS, Kazzi B, Varma B, Choi E, Thakkar A, Michos ED. Sex-specific differences in cardiovascular risk factors and implications for cardiovascular disease prevention in women. Atherosclerosis. 2023;384:117269.
doi pubmed - Farooq V, van Klaveren D, Steyerberg EW, Meliga E, Vergouwe Y, Chieffo A, Kappetein AP, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet. 2013;381(9867):639-650.
doi pubmed - Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596-e646.
doi pubmed - Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16(3):177-189.
doi pubmed - Powell-Wiley TM, Baumer Y, Baah FO, Baez AS, Farmer N, Mahlobo CT, Pita MA, et al. Social determinants of cardiovascular disease. Circ Res. 2022;130(5):782-799.
doi pubmed - Cui C, Liu L, Qi Y, Han N, Xu H, Wang Z, Shang X, et al. Joint association of TyG index and high sensitivity C-reactive protein with cardiovascular disease: a national cohort study. Cardiovasc Diabetol. 2024;23(1):156.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.