| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 1, February 2025, pages 44-52
Relationship Between Advanced Glycation End Products Tissue Accumulation and Frailty in Patients Undergoing Cardiac Rehabilitation
Hiroki Kasuyaa, Miho Nishitani-Yokoyamaa, c, Mitsuhiro Kunimotoa, Kei Fujiwaraa, Jianying Xua, Abidan Abulimitia, Yurina Sugita-Yamaguchia, Kazunori Shimadaa, Hiroyuki Daidaa, Minoru Tabatab, Tohru Minaminoa
aDepartment of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine, Bunkyo-ku, Tokyo 113-8421, Japan
bDepartment of Cardiovascular Surgery, Juntendo University Graduate School of Medicine, Bunkyo-ku, Tokyo 113-8421, Japan
cCorresponding Author: Miho Nishitani-Yokoyama, Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine, Bunkyo-ku, Tokyo 113-8421, Japan. Bunkyo-ku, Tokyo 113-8421, Japan
Manuscript submitted November 15, 2024, accepted January 15, 2025, published online January 21, 2025
Short title: Association Between AGEs and Frailty in CR
doi: https://doi.org/10.14740/cr2010
| Abstract | ▴Top |
Background: The advanced glycation end products (AGEs), which can be assessed through skin autofluorescence (SAF), have been linked to chronic kidney disease (CKD), diabetes mellitus (DM), and aging. However, it is unknown how frailty and SAF levels are associated with cardiovascular disease (CVD).
Methods: We enrolled 1,000 consecutive CVD patients who participated in phase II cardiac rehabilitation (CR) and underwent assessment of SAF between November 2015 and September 2017 at Juntendo University Hospital. Of these, 48 patients were excluded as duplicate cases, and a deficiency in SAF data led to the exclusion of an additional 146 patients. The final analysis included 806 patients.
Results: Seventy percent of patients were male, and the mean age was 67.0 ± 12.9 years. In this study, the patients were divided into two groups (high SAF group and low SAF group) based on the median SAF level (2.9 a.u.), which is known as a cutoff value to increase the risk of CVD in previous studies. Compared with the low SAF group (n = 368, 45.7%), the high SAF group (n = 438; 54.3%) was older, and the Kihon Checklist (KCL) total score and prevalence of DM and CKD were significantly higher (all, P < 0.05). Multivariate regression analyses demonstrated that age was the only independent associated factor (P < 0.05) in the low SAF group. Conversely, in the high SAF group, creatinine, hemoglobin A1c (HbA1c) and the sub-total KCL score (1 - 20) were independently associated with SAF levels (all, P < 0.05).
Conclusions: Frailty assessed by KCL is one of the factors significantly correlated with the accumulation of AGEs as well as creatinine, HbA1c and brain natriuretic peptide (BNP) levels in the high SAF group of patients with CVD undergoing phase II CR, who have the higher risk of the onset of CVD and all-cause mortality.
Keywords: Advanced glycation end products; Cardiac rehabilitation; Cardiovascular disease, Frailty
| Introduction | ▴Top |
Advanced glycation products (AGEs) are harmful compounds formed by proteins, lipids, and nucleic acids [1]. Smoking, unhealthy diets which make the chemical transformation of amine-containing molecules by reducing sugars, and aging contribute to the accumulation of AGEs in the body. This accumulation is accelerated by conditions such as hyperglycemia, inflammation, and oxidative stress [2, 3].
Traditionally, enzyme-linked immunosorbent assays and other methods have been used to measure AGEs for circulating and tissue-bound AGEs [4]. It is also known that tissue-bound AGEs are more stable than circulating AGEs, which have greater physiological variability. In particular, tissue-bound AGEs in slow-turnover tissues, such as the epidermis, vascular wall, and eyeball, are suitable for AGE measurement because of their high accumulation [5]. Although skin is one of the most accessible tissues to measure AGEs, it still required invasive, expensive, and time-consuming analysis, as the golden standard for performing biopsy and biochemical analysis. However, the clinical application of AGEs measurement has recently become feasible with the development of a device that measures skin autofluorescence (SAF), a straightforward, noninvasive, and accurate method for assessing AGEs accumulation in the skin [6].
Some previous research has demonstrated an association between AGEs and aging, diabetes mellitus (DM), and chronic kidney disease (CKD) [7]. Moreover, it has been discovered that SAF levels predict the risk of the onset of cardiovascular disease (CVD) and all-cause mortality [8-11]. Because of cumulative losses in several physiological systems, frailty is thought to be a biological syndrome characterized by diminished reserve and resistance to stressors, making people susceptible to negative outcomes, such as poor lifestyle choices, the need for nursing care, and even death. Apart from medical issues, the term “frailty” also refers to mental health and psychological issues, including depression and cognitive impairment, as well as social issues, such as living alone and financial hardship [12].
As the population ages, the prevalence of CVD and lifestyle-related diseases continues to rise [13, 14]. However, there is limited research on the relationship between AGEs accumulation and physical frailty in patients with CVD. This study aims to use SAF levels to assess the association between AGEs accumulation and clinical indicators, including frailty characteristics, in patients with CVD undergoing cardiac rehabilitation (CR).
| Materials and Methods | ▴Top |
Study population
In this retrospective observational analysis, 1,000 consecutive patients with CVD who undergoing phase II CR at Juntendo University Hospital in Tokyo, Japan, between November 2015 and September 2017 were included. CR is a comprehensive program for secondary CVD prevention that improves the long-term prognosis, and it is covered by insurance for 150 days from the acute phase (phase I) to the late recovery phase (phase II) stage [15]. Patients who had completed early phase II CR and stable cardiac states were enrolled in this trial. Of these, 48 patients were excluded as duplicate cases, and a deficiency in SAF data led to the exclusion of an additional 146 patients. The final analysis included 806 patients (Fig. 1). Before participation, all patients provided written, informed consent. The study protocol followed the Declaration of Helsinki and was approved by our institution’s ethics committee (CRB3180012). Trial ID jRCTs032180156 was used to register this study in the Japan Registry of Clinical Trials (jRCT) database.
 Click for large image | Figure 1. Flow diagram for enrolling patients. Phase II CR was performed on 1,000 consecutive patients with CVD. Of these, 48 patients were excluded as duplicate cases, and an additional 146 patients were not included because of insufficient SAF data. The final analysis included 806 patients. CR: cardiac rehabilitation; SAF: skin autofluorescence. |
Data collection and measurements
The patient’s medical records were used to determine age, gender, smoking history, comorbidities, and medical history. The hemoglobin A1c (HbA1c) level of 6.5 or above or DM medication was required to diagnose the disease. The Japanese Society of Nephrology defined the estimated glomerular filtration rate (eGFR) for CKD as < 60 mL/min/1.73 m2 [16]. Following a nighttime fast (over 9 h), blood samples were obtained early in the morning.
At the beginning of phase II CR, measurements were made of body composition, grip strength, and SAF levels [17]. A bioelectrical impedance analysis (TANITA, MC-780 A, Tokyo, Japan) was used to quantify body composition parameters, including body fat percentage, lean body mass, and muscle mass.
SAF
While the individual was seated, SAF levels were measured using an AGE Reader (DiagnOptics Technologies B.V., Groningen, Netherlands) on the upper arm. The AGE Reader is a recently developed device that can easily and noninvasively measure the degree of photoexcited fluorescence, which can be used to precisely assess the accumulation of AGEs in the skin [7, 18, 19]. SAF levels are known to be described as a linear function of age up to age of 70 years [4]. And it is also known that CVD risk is significantly increased at SAF levels of 2.9 or higher in any age group [18]. Autofluorescence represents the spontaneous emission of the substrate after absorbing light [7]. Autofluorescence is also observed in many redox-regulated fluorophores, including reduced nicotinamide adenine dinucleotide (NADH), flavin-adenine dinucleotide, and porphyrin [18, 19]. However, approximately 76% of the variation in SAF levels can be explained by variations in the concentration of pentosidine, the major AGE in the skin [20]. Therefore, SAF levels are based on the autofluorescent properties of some AGEs, including pentosidine [21]. Pentosidine has an excitation wavelength of 325 - 335 nm and an emission wavelength of 375 - 385 nm. Meanwhile, the SAF levels were calculated by the AGE Reader as the ratio of the average excitation light intensity in the 300 - 420 nm and the average light intensity in the 420 - 600 nm wavelength ranges. Thus, the AGE Reader measures SAF levels as part of the fluorescence spectrum of pentosidine; this depicts the accumulation of AGEs between the skin’s dermis and epidermis.
Kihon Checklist (KCL)
The Japanese Ministry of Health, Labor and Welfare created the KCL, a questionnaire designed to identify vulnerable elderly people needing long-term support or medical attention under a long-term healthcare insurance system [22]. The 25 questions on the KCL are self-administered and cover the following topics: daily living activities (three questions), social activities of daily living (four questions), physical function (five questions), nutritional status (two questions), oral function (three questions), cognitive function (three questions) and depressive mood (five questions). All questions in the KCL comprise two yes-or-no questions, each of which is counted with a score of 1 or 0. The physical, psychological, functional, and social status of older persons without disabilities can be evaluated in many dimensions using this extensive questionnaire. A high score in any checklist domain denotes a high likelihood of requiring support or care in that area. The Cardiovascular Health Study (CHS) frailty index, as reported by Fried et al, is a currently used and highly accepted tool for evaluating frailty [12]. In previous studies, the KCL has been shown to correlate significantly with the CHS frailty index, with a KCL score of ≥ 8 indicating frailty and 4 - 7 indicating prefrailty [23]. Also, subtotal scores of 1 - 20, excluding the mood domain of the KCL, are shown to have a high correlation with frailty, indicating that a subscore of ≥ 6 is considered frailty [24].
Statistical analysis
Welch’s t-test was used for continuous variables, mean ± standard deviation was used for normally distributed values, median (interquartile range) was used for non-normally distributed variables, and Chi-squared test was used for categorical data to assess group comparison. To investigate the correlation between the SAF level and additional variables, univariate analysis was employed. Among the factors that were found to be significant in this univariate analysis, the stepwise method was used in order to explore factors related to the amount of change in SAF levels. We performed those analysis for all patients, low SAF level group and high SAF level group, respectively. When P < 0.05 was reached, the differences were deemed statistically significant. Statistical analyses were performed using JMP version 17.0 (SAS Institute, Cary, NC, USA).
| Results | ▴Top |
SAF data and baseline attributes
A total of 806 patients were included for the examination. There were 570 (70.7%) males, with a mean age of 67.0 ± 12.9 years (Table 1). The distribution of the SAF levels is displayed in Figure 2. The SAF levels were 3.0 ± 0.6 a.u. for the mean and 2.9 a.u. for the median (interquartile range: 2.5 - 3.3 a.u.). This median level was consistent with the significant threshold for increased CVD risk development, as noted above. Therefore, the patients were divided into the low (< 2.9 a.u., n = 368) and the high (≥ 2.9 a.u., n = 438) SAF groups.
 Click to view | Table 1. The Comparison of Clinical Characteristics of Patients Between the High and Low SAF Groups |
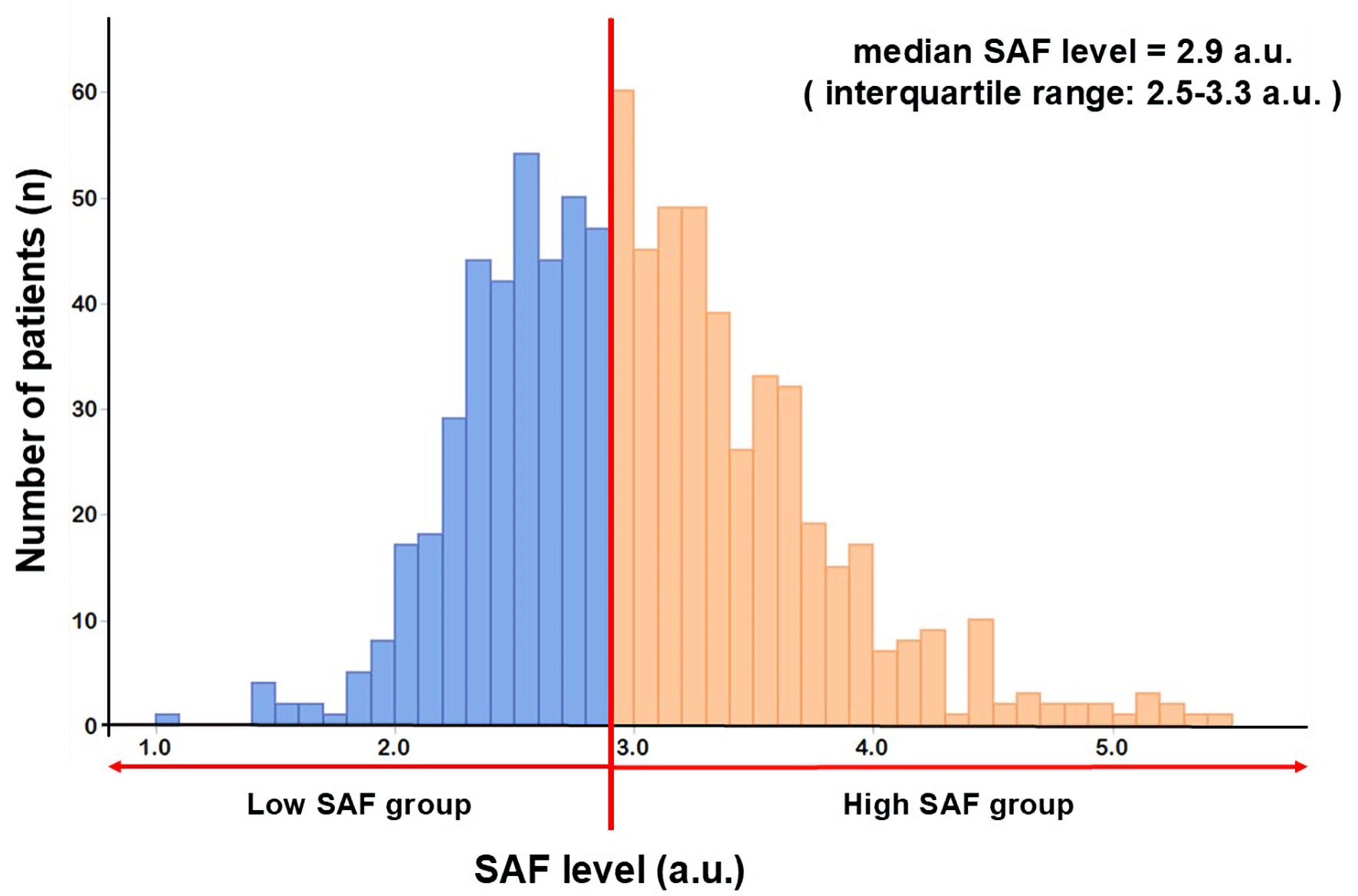 Click for large image | Figure 2. SAF level distribution. The SAF levels were 3.0 ± 0.6 a.u. for the mean and 2.9 a.u. for the median (interquartile range: 2.5 - 3.3 a.u.). The Shapiro-Wilk test of normality: P < 0.05. SAF: skin autofluorescence. |
Comparing the groups with high and low SAF levels
Table 1 shows the comparison of the clinical parameters between the two groups. The sex ratio did not significantly differ between the two groups. Compared with the low SAF group, the high SAF group was significantly older (70.9 ± 10.6 vs. 62.4 ± 13.7 years old, P < 0.01) and had a significantly higher DM prevalence (39.7% vs. 24.2%, P < 0.01), CKD (40.4% vs. 25.6%, P < 0.01), body fat percentage (23.4 ± 9.0% vs. 21.9 ± 8.6%, P = 0.03), creatinine (0.9 (0.7 - 1.1)) vs. 0.8 (0.6 - 0.9) mg/dL, P < 0.01) , HbA1c (6.1±1.0% vs. 5.9±0.8 %, P < 0.01), and brain natriuretic peptide (BNP) (100.1 (42.2 - 286.0) vs. 71.6 (27.7 - 177.2)) pg/mL, P < 0.01) levels. Furthermore, the prescription rate of statins (58.8% vs. 45.8%, P < 0.01), oral hypoglycemic agents (21.7% vs. 9.3%, P < 0.01), insulin (10.3% vs. 1.6%, P < 0.01), and total score of KCL (6.2 ± 4.3 vs. 5.5 ± 4.1, P = 0.02) were significantly higher. However, in the low SAF group, the quantity of patients suffering from aortic disease (5.7% vs. 9.8%, P = 0.03), lean body mass (46.1 ± 9.1 vs. 47.1 ± 10.2 kg, P = 0.04), grip strength (28.1 ± 8.4 vs. 29.8 ± 9.5 kg, P = 0.03), hemoglobin (12.7 ± 1.9 vs. 13.4 ± 1.9 g/dL, P < 0.01), albumin level (3.8 ± 0.5 vs. 3.9 ± 0.5 g/dL, P < 0.01), and eGFR (64.0 ± 28.2 vs. 75.3 ± 23.9 mL/min/1.73m2, P < 0.01) were significantly higher.
Correlation between SAF levels and clinical parameters
The correlation between SAF levels and clinical parameters is shown in Table 2. Significant correlations were observed between SAF levels and age, gender, body mass index, level of hemoglobin, albumin, creatinine, eGFR, lipid profiles, HbA1c and BNP. Furthermore, there were significant differences in both total and sub-total KCL score and the prevalence of frailty and prefrailty calculated by them. Next, we examined the relationship between clinical measures and SAF levels in the high and low SAF groups. In the high SAF group, SAF levels were associated with age, level of hemoglobin, albumin, creatinine, eGFR, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), HbA1c, and BNP. In addition, there were significant differences in both total and sub-total KCL score and the prevalence of frailty and prefrailty calculated by them. In the low SAF group, SAF levels were associated with age, and level of albumin and eGFR.
 Click to view | Table 2. Correlation Between SAF Levels and Clinical Parameters |
Multivariate analysis of SAF levels
In all patients, multiple regression analysis with clinical parameters correlated with SAF levels showed that age, BMI, creatinine, HbA1c and BNP were significantly associated with SAF levels (β = 0.54/0.19/0.80/0.45/0.51, all P < 0.05). In the low SAF group, age was the only factor significantly associated with SAF levels (β = 0.16, P < 0.01). However, in the high SAF group, creatinine, HbA1c, BNP (β = 0.56/ 0.35/ 0.48, all P < 0.05), and the sub-total score of KCL (β = 0.15, P = 0.01) were independently associated with SAF levels (Table 3).
 Click to view | Table 3. Multivariate Regression Analysis With Clinical Parameters Correlated SAF Levels in All Patients and the High and Low SAF Groups |
| Discussion | ▴Top |
The present study assessed SAF levels in patients with CVD undergoing phase II CR and examined the relationship between SAF levels and clinical background characteristics, such as frailty. In patients with CVD undergoing CR, especially in the high SAF group, we found that frailty assessed by KCL was significantly correlated with AGEs which were measured by SAF levels, HbA1c, renal function, and BNP. This is the first report of an association between SAF levels measured on the forearm and frailty in patients with CVD undergoing phase II CR. In this study, the median SAF level was 2.9 a.u. (interquartile range: 2.5 - 3.3 a.u.), which is consistent with the boundary value for increased risk of developing CVD already shown in previous studies [18]. The present study also showed that, in the low SAF group, age was the only factor independently associated with SAF levels. It is well recognized that age contributes significantly to the accumulation of AGEs in all organs, which increases linearly with age in healthy subjects under 70 years of age and is even higher in those over 70 [4]. In addition, in the high SAF group, sub-total scores of KCL, creatinine, HbA1c and BNP levels were significantly independent factors associated with SAF levels. It is well established that individuals with diabetes have greater SAF levels than healthy individuals. It is known that the accumulation of AGEs is a predictor of the onset and progression of DM. In previous studies, the SAF levels of DM subjects without complications was 2.56 a.u. (interquartile range: 2.26 - 2.90 a.u.), whereas the SAF levels of DM subjects with microvascular complications, such as microalbuminuria, retinopathy, and neuropathy, was 2.79 a.u. (interquartile range: 2.38 - 3.29 a.u.). Moreover, the SAF levels of DM subjects with cardiovascular or cerebrovascular disease were 2.85 a.u. (interquartile range: 2.41 - 3.41 a.u.), and those with both were 2.96 a.u. (interquartile range: 2.56 - 3.60 a.u.) [25]. Thus, according to reports, the accumulation of AGEs in patients with DM with complications is significantly higher than in those without complications. According to other previous research, the buildup of AGEs is significantly correlated with age, renal function, and DM [26, 27]. Furthermore, we have demonstrated in a previous study that accumulation of AGE is substantially linked with age, HbA1c, and renal function in patients with CVD undergoing CR [28]. Although no previous studies have shown a direct relationship between the accumulation of AGEs and BNP levels, it has been reported that pentosidine, a representative component of AGEs, was high in patients with heart failure, and the degree of elevation correlated with the severity of heart failure [29]. In this study, the high SAF group had significantly higher BNP levels than those of the low SAF group, although there were no significant differences in the distribution of CVD at the initiation of CR. Therefore, BNP levels, a useful indicator of controlling heart failure, were considered one of the independent factors associated with SAF levels, representing the amount of pentosidine accumulation. The binding of AGEs to AGE receptors activates inflammatory responses and oxidative stress pathways, including interleukin (IL)-6, and this response has also been suggested to increase frailty [30, 31]. In addition, we have demonstrated a correlation between exercise tolerance and the accumulation of AGEs in patients undergoing CR [28]. These reports may support the present association between AGEs and frailty, especially the KCL subscore. However, there are some limitations to this study. First, this was a cross-sectional, single-center study. Therefore, it is difficult to prove causality in this study alone. Second, patients with CVD were included in the study under phase II CR. Therefore, it is difficult to discuss this study as a patient with general CVD. Third, the study is based on the accumulation of AGEs obtained by the SAF level on the skin surface, which does not measure all AGEs in the body.
Conclusions
Frailty assessed by KCL is one of the factors significantly correlated with the accumulation of AGEs, which is increased by oxidative stress and the progression of atherosclerosis, as well as creatinine, HbA1c and BNP levels, in the high SAF group of patients with CVD undergoing phase II CR, who have the higher risk of the onset of CVD and all-cause mortality.
Acknowledgments
The authors wish to thank all study participants and members of the data collection committee at the Cardiovascular Rehabilitation and Fitness and Clinical Translational Science, Juntendo University Graduate School of Medicine, Tokyo, Japan.
Financial Disclosure
This work was supported by JSPS KAKENHI (Grant No. JP22K11428).
Conflict of Interest
All authors declare no conflict of interest.
Informed Consent
Before participation, all patients provided written, informed consent according to the ethics committee of Juntendo University Hospital.
Author Contributions
HK and MY designed the study. MK, KF and YY conducted the research. JX and AA assisted with sample collection and statistical analysis. KS, HD, MT and TM carefully supervised the manuscript preparation and writing.
Data Availability
Participant data has been deidentified and will not be distributed. The database analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
AGEs: advanced glycation end products; SAF: skin autofluorescence; CKD: chronic kidney disease; DM: diabetes mellitus; CVD: cardiovascular disease; CR: cardiac rehabilitation; KCL: Kihon Checklist; HbA1c: hemoglobin A1c; eGFR: estimated glomerular filtration rate; NADH: nicotinamide adenine dinucleotide; CHS: Cardiovascular Health Study; BNP: brain natriuretic peptide; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol, BMI, body mass index; CHF: chronic heart failure; AMI: acute myocardial infarction; AP: angina pectoris; LVEF: left ventricle ejection fraction; TG: triglyceride; ACE-I: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker
| References | ▴Top |
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813-820.
doi pubmed - Prasad K, Dhar I, Caspar-Bell G. Role of advanced glycation end products and its receptors in the pathogenesis of cigarette smoke-induced cardiovascular disease. Int J Angiol. 2015;24(2):75-80.
doi pubmed - Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14(1):453.
doi pubmed - Mulder DJ, Water TV, Lutgers HL, Graaff R, Gans RO, Zijlstra F, Smit AJ. Skin autofluorescence, a novel marker for glycemic and oxidative stress-derived advanced glycation endproducts: an overview of current clinical studies, evidence, and limitations. Diabetes Technol Ther. 2006;8(5):523-535.
doi pubmed - Lyons TJ, Bailie KE, Dyer DG, Dunn JA, Baynes JW. Decrease in skin collagen glycation with improved glycemic control in patients with insulin-dependent diabetes mellitus. J Clin Invest. 1991;87(6):1910-1915.
doi pubmed - Meerwaldt R, Graaff R, Oomen PHN, Links TP, Jager JJ, Alderson NL, Thorpe SR, et al. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47(7):1324-1330.
doi pubmed - Koetsier M, Lutgers HL, de Jonge C, Links TP, Smit AJ, Graaff R. Reference values of skin autofluorescence. Diabetes Technol Ther. 2010;12(5):399-403.
doi pubmed - Yamagishi S, Fukami K, Matsui T. Evaluation of tissue accumulation levels of advanced glycation end products by skin autofluorescence: A novel marker of vascular complications in high-risk patients for cardiovascular disease. Int J Cardiol. 2015;185:263-268.
doi pubmed - Cavero-Redondo I, Soriano-Cano A, Alvarez-Bueno C, Cunha PG, Martinez-Hortelano JA, Garrido-Miguel M, Berlanga-Macias C, et al. Skin autofluorescence-indicated advanced glycation end products as predictors of cardiovascular and all-cause mortality in high-risk subjects: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7(18):e009833.
doi pubmed - van Waateringe RP, Fokkens BT, Slagter SN, van der Klauw MM, van Vliet-Ostaptchouk JV, Graaff R, Paterson AD, et al. Skin autofluorescence predicts incident type 2 diabetes, cardiovascular disease and mortality in the general population. Diabetologia. 2019;62(2):269-280.
doi pubmed - Fukushima Y, Daida H, Morimoto T, Kasai T, Miyauchi K, Yamagishi S, Takeuchi M, et al. Relationship between advanced glycation end products and plaque progression in patients with acute coronary syndrome: the JAPAN-ACS sub-study. Cardiovasc Diabetol. 2013;12:5.
doi pubmed - Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-156.
doi pubmed - Uchikado Y, Ikeda Y, Ohishi M. Current understanding of the role of frailty in cardiovascular disease. Circ J. 2020;84(11):1903-1908.
doi pubmed - Aili SR, Lo P, Villanueva JE, Joshi Y, Emmanuel S, Macdonald PS. Prevention and reversal of frailty in heart failure - a systematic review. Circ J. 2021;86(1):14-22.
doi pubmed - Makita S, Yasu T, Akashi YJ, Adachi H, Izawa H, Ishihara S, Iso Y, et al. JCS/JACR 2021 guideline on rehabilitation in patients with cardiovascular disease. Circ J. 2022;87(1):155-235.
doi pubmed - Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982-992.
doi pubmed - Nishitani-Yokoyama M, Miyauchi K, Shimada K, Yokoyama T, Ouchi S, Aikawa T, Kunimoto M, et al. Impact of physical activity on coronary plaque volume and components in acute coronary syndrome patients after early phase II cardiac rehabilitation. Circ J. 2018;83(1):101-109.
doi pubmed - Stirban A, Heinemann L. Skin autofluorescence - a non-invasive measurement for assessing cardiovascular risk and risk of diabetes. Eur Endocrinol. 2014;10(2):106-110.
doi pubmed - Kato M, Kubo A, Sugioka Y, Mitsui R, Fukuhara N, Nihei F, Takeda Y. Relationship between advanced glycation end-product accumulation and low skeletal muscle mass in Japanese men and women. Geriatr Gerontol Int. 2017;17(5):785-790.
doi pubmed - Smit AJ, Gerrits EG. Skin autofluorescence as a measure of advanced glycation endproduct deposition: a novel risk marker in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19(6):527-533.
doi pubmed - Lutgers HL, Graaff R, Links TP, Ubink-Veltmaat LJ, Bilo HJ, Gans RO, Smit AJ. Skin autofluorescence as a noninvasive marker of vascular damage in patients with type 2 diabetes. Diabetes Care. 2006;29(12):2654-2659.
doi pubmed - Arai H, Satake S. English translation of the Kihon Checklist. Geriatr Gerontol Int. 2015;15(4):518-519.
doi pubmed - Satake S, Senda K, Hong YJ, Miura H, Endo H, Sakurai T, Kondo I, et al. Validity of the Kihon Checklist for assessing frailty status. Geriatr Gerontol Int. 2016;16(6):709-715.
doi pubmed - Ogawa K, Fujiwara Y, Yoshida H, Nishi M, Fukaya T, Kim M, Amano H, et al. [The validity of the "Kihon Check-list" as an index of frailty and its biomarkers and inflammatory markers in elderly people]. Nihon Ronen Igakkai Zasshi. 2011;48(5):545-552.
doi pubmed - Noordzij MJ, Mulder DJ, Oomen PH, Brouwer T, Jager J, Castro Cabezas M, Lefrandt JD, et al. Skin autofluorescence and risk of micro- and macrovascular complications in patients with Type 2 diabetes mellitus-a multi-centre study. Diabet Med. 2012;29(12):1556-1561.
doi pubmed - Friedman EA. Advanced glycosylated end products and hyperglycemia in the pathogenesis of diabetic complications. Diabetes Care. 1999;22(Suppl 2):B65-71.
pubmed - Henle T, Miyata T. Advanced glycation end products in uremia. Adv Ren Replace Ther. 2003;10(4):321-331.
doi pubmed - Kunimoto M, Shimada K, Yokoyama M, Matsubara T, Aikawa T, Ouchi S, Shimizu M, et al. Association between the tissue accumulation of advanced glycation end products and exercise capacity in cardiac rehabilitation patients. BMC Cardiovasc Disord. 2020;20(1):195.
doi pubmed - Koyama Y, Takeishi Y, Arimoto T, Niizeki T, Shishido T, Takahashi H, Nozaki N, et al. High serum level of pentosidine, an advanced glycation end product (AGE), is a risk factor of patients with heart failure. J Card Fail. 2007;13(3):199-206.
doi pubmed - Teissier T, Boulanger E. The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging. Biogerontology. 2019;20(3):279-301.
doi pubmed - Butcher L, Carnicero JA, Gomez Cabrero D, Dartigues JF, Peres K, Garcia-Garcia FJ, Rodriguez-Manas L, et al. Increased levels of soluble Receptor for Advanced Glycation End-products (RAGE) are associated with a higher risk of mortality in frail older adults. Age Ageing. 2019;48(5):696-702.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.