| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 2, April 2025, pages 140-152
Effect of Dapagliflozin on Ventricular Arrhythmic Events in Heart Failure Patients With an Implantable Cardioverter Defibrillator
Gabriele De Masi De Lucaa, b, c, h , Zefferino Palamaa, d, Simonetta Longob, Francesca Barbab, Antonio Gianluca Roblesa, e, Martina Nestif, Antonio Scarag, Giovanni Colucciab, Marzia Colopib, Giuseppe De Masi De Lucac, Simona Minardia, Liuba Fuscoa, Pietro Palmisanob, Michele Accoglib, Luigi Sciarraa, Silvio Romanoa
aDepartment of Life, Health and Environmental Science, University of L’Aquila, L’Aquila, Italy
bCardiology Unit, Card. “G. Panico” Hospital, Tricase, Italy
cCardiomed Medical Center, Maglie, Italy
dCardiology Unit, “Villa Verde” Hospital, Taranto, Italy
eCardiology Department, Ospedale “L. Bonomo”, Andria, Italy
fCardiology Unit, CNR Fondazione Toscana “Gabriele Monasterio”, Pisa, Italy
gGVM Care and Research, “San Carlo di Nancy” Hospital, Rome, Italy
hCorresponding Author: Gabriele De Masi De Luca, Cardiology Unit, Card. “G. Panico” Hospital, Tricase, Italy
Manuscript submitted November 24, 2024, accepted January 21, 2025, published online February 18, 2025
Short title: Effect of DAPA on Vab in HF Patients With ICD
doi: https://doi.org/10.14740/cr2018
| Abstract | ▴Top |
Background: The aim of our study was to evaluate the effects of dapagliflozin on the ventricular arrhythmia burden (VAb) in patients with heart failure with reduced ejection fraction (HFrEF) and an implantable cardioverter defibrillator (ICD), correlating the possible reduction in arrhythmic events and ICD therapies with the basal functional capacity, as well as the remodeling parameters induced by treatment.
Methods: A total of 117 outpatient ICD patients with a known diagnosis of HFrEF who underwent treatment with dapagliflozin were evaluated according to a prospective observational protocol. VAb (including sustained ventricular tachycardia, non-sustained ventricular tachycardia, ventricular fibrillation, and total ventricular events) and specific ICD therapies (anti-tachycardia pacing (ATP) and ICD shocks) were extrapolated from the devices’ memory (events per patient per month) by comparing events in the observation period before and after the introduction of dapagliflozin.
Results: The VAb was significantly reduced after dapagliflozin introduction (2.9 ± 1.8 vs. 4.5 ± 2.0, P = 0.01). The burden of appropriate ATPs was significantly reduced (0.57 ± 0.80 vs. 0.65 ± 0.91, P = 0.03), but not for ICD shocks. In patients with a more advanced functional class, a greater reduction in VAb was observed than in patients with a better initial functional capacity (2.2 ± 0.8 vs. 5.5 ± 1.8, P = 0.001 in the New York Heart Association (NYHA) III/IV group; 3.5 ± 2.1 vs. 4.5 ± 2.2, P = 0.02 in the NYHA I/II group). Considering two independent groups according to reverse remodeling (Δleft ventricular ejection fraction (LVEF) > 15%), a significant reduction in VAb was observed only in those patients who presented significant reverse remodeling (2.5 ± 1.1 vs. 5.1 ± 1.6, P = 0.01). A statistically significant interaction between the variation of total ventricular arrhythmias (VTA) and the basal NYHA class (F(1,115) = 142.25, P < 0.0001, partial η2 = 0.553), as well as between the variation of VTA and the ΔLVEF (F(1,115) = 107.678, P < 0.0001, partial η2 = 0.484) has been demonstrated using a two-way analysis of variance (ANOVA) test.
Conclusions: In ICD outpatients with HFrEF, dapagliflozin treatment produces a reduction in arrhythmic ventricular events. This improvement is more evident in patients who have a worse functional class and thus a more precarious hemodynamic state, and in patients who present with significant ventricular reverse remodeling. Therefore, we can hypothesize that the hemodynamic and structural improvements induced by treatment represent, at least in the short-medium term, some of the principal elements justifying the significant reduction in VAb.
Keywords: Dapagliflozin; SGLT2; Heart failure; Ventricular arrhythmic burden; Global longitudinal strain; ICD therapies
| Introduction | ▴Top |
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a class of drugs that have been shown to reduce the worsening of heart failure (HF) and death from cardiovascular causes in diverse types of HF, thus indicating that they should enter the therapeutic armamentarium in addition to the other classes of drugs that are already present in recent guidelines [1-4].
Several studies have shown that the use of sodium-glucose cotransporter 2 inhibitors (SGLT2is) is associated with a reduction in atrial and ventricular arrhythmic events [5, 6]. The data from the DAPA-HF study showed that the occurrence of the composite endpoint of severe ventricular arrhythmic events, resuscitated cardiac arrest, and sudden death was significantly reduced in patients receiving dapagliflozin compared to the placebo group [5].
The burden of cardiac arrhythmias is still a matter of debate, especially in the field of ventricular burden. Several potential antiarrhythmic actions related to SGLT2 inhibitors have been hypothesized. Various proposed actions of SGLT2 inhibitors lead to the hypothesis that these agents might reduce the risk of ventricular arrhythmias. Among the main indirect antiarrhythmic effects that have already been amply studied are the improvement in hemodynamic status and the structural remodeling induced by the treatment [7-11]. The potential direct antiarrhythmic actions include favorable effects on the autonomic nervous system activity, serum electrolytes, cardiac sodium channel current (late/Na+), and myocardial sodium-hydrogen (Na+/H+) exchanger, thereby improving mitochondrial function and reducing electrical instability by ensuring a sufficient energy supply [12-19].
The aim of our study was to evaluate the effects of dapagliflozin on the ventricular arrhythmia burden (VAb) in patients with cardiac implantable electronic devices, correlating with a possible reduction in arrhythmic events with the basal clinical status and with the trend of treatment-induced ventricular remodeling.
| Materials and Methods | ▴Top |
Study population
According to a prospective observational longitudinal protocol, this study evaluated 117 outpatient implantable cardioverter defibrillator (ICD) patients with a known diagnosis of HF within a dedicated follow-up pathway at the “Card. Panico” Hospital in Tricase (Le) and “Villa Verde” Hospital in Taranto from March 2023 to November 2023. The present study was conducted in accordance with the Declaration of Helsinki and carried out within the research project approved by the Institutional Ethics Committee.
We evaluated ICD patients with heart failure with reduced ejection fraction (HFrEF), who were receiving optimal pharmacological therapy, with 10 mg dapagliflozin introduced. Patients who, during the observation period, underwent changes in their cardiology therapy (including changes in antiarrhythmic drugs and diuretic dosage), experienced respiratory instability or renal failure, and experienced any hospitalization for HF or electrolyte disorder, were excluded from the evaluation. No acute anemic states were observed during the evaluation period, and there was no evidence of thyroid dysfunction. No patients received cardiac resynchronization therapy (CRT) treatment during the observation period (Fig. 1).
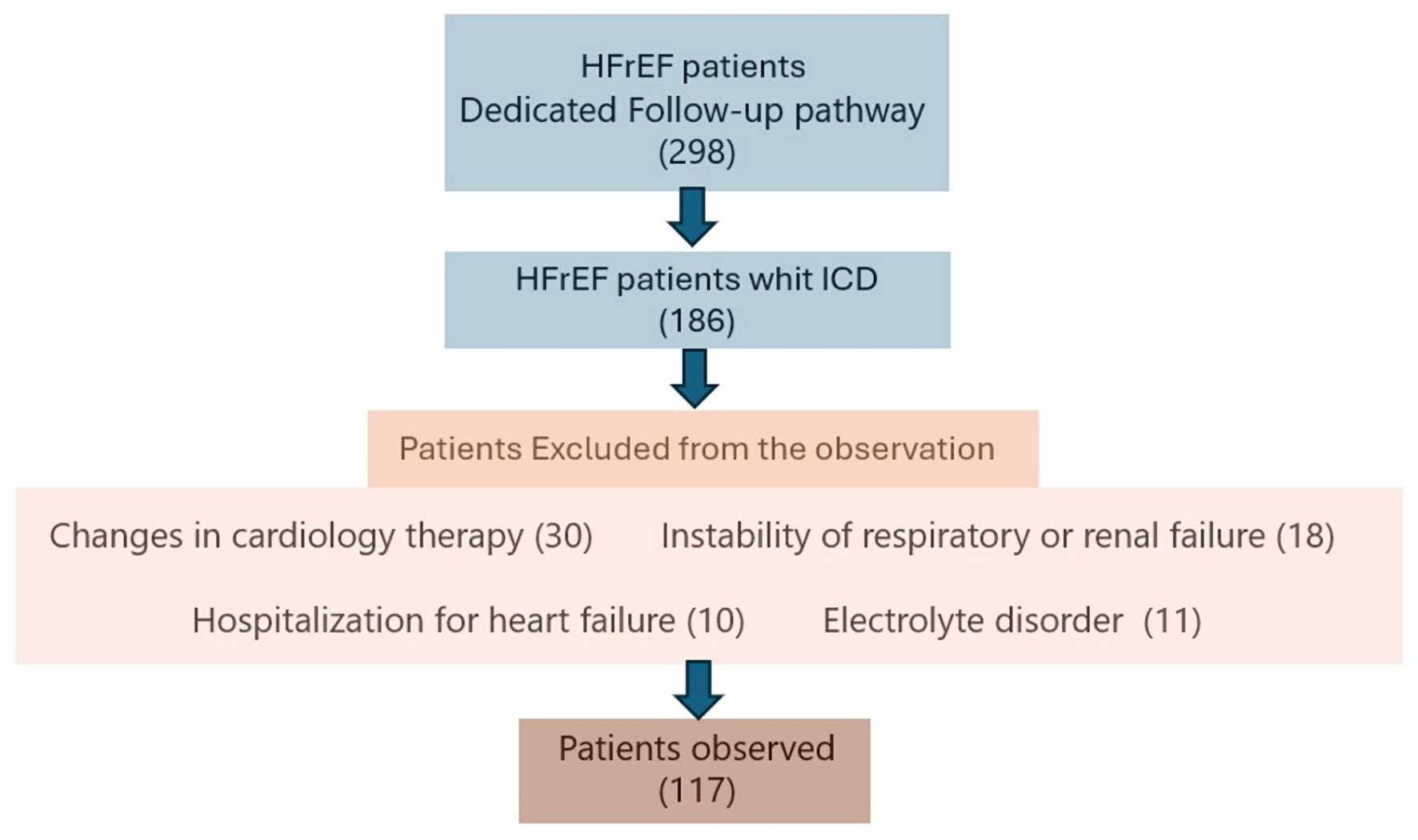 Click for large image | Figure 1. Inclusion and exclusion criteria for the patients’ identification. ICD: implantable cardioverter defibrillator. |
The anamnestic, clinic, and echocardiographic parameters were extrapolated from the hospital management system with an embedded database. The New York Heart Association (NYHA) functional class, echocardiographic parameters such as left ventricular ejection fraction (LVEF), ventricular filling index (E/E’), global longitudinal strain (GLS), pulmonary arterial systolic pressure (PASP), and N-terminal B-type natriuretic peptide (NT-proBNP), which were obtained at baseline and 6 months after the dapagliflozin introduction, were considered. Echocardiographic studies were obtained locally by the same accredited specialists at each center, using the same equipment according to the standardized quality criteria for each protocol. All of the echocardiographic examinations were performed with the same echocardiographic machines (Philips Affinity 50, Andover, MA, USA) and speckle tracking analysis with dedicated software (QLAB13/TOMTEC Philips), according to the echocardiographic guidelines of the European Association of Cardiovascular Imaging (EACVI) [20]. An improvement in the LVEF of at least 15% (ΔLVEF) was considered as a parameter indicative of ventricular reverse remodeling when comparing the data obtained at baseline and at the 6-month follow-up. Some studies have shown that dapagliflozin produces an improvement in systolic function indices as early as 6 months after the start of treatment. Therefore, we chose to evaluate ventricular remodeling by comparing the baseline echocardiographic parameters with the data from 6-month assessments [21, 22] (Fig. 2). Considering the noticeable improvement of the left ventricular systolic function that was obtained during the follow-up, we chose a more aggressive cut-off value of ΔLVEF to make the sample groups’ numerosity more homogeneous.
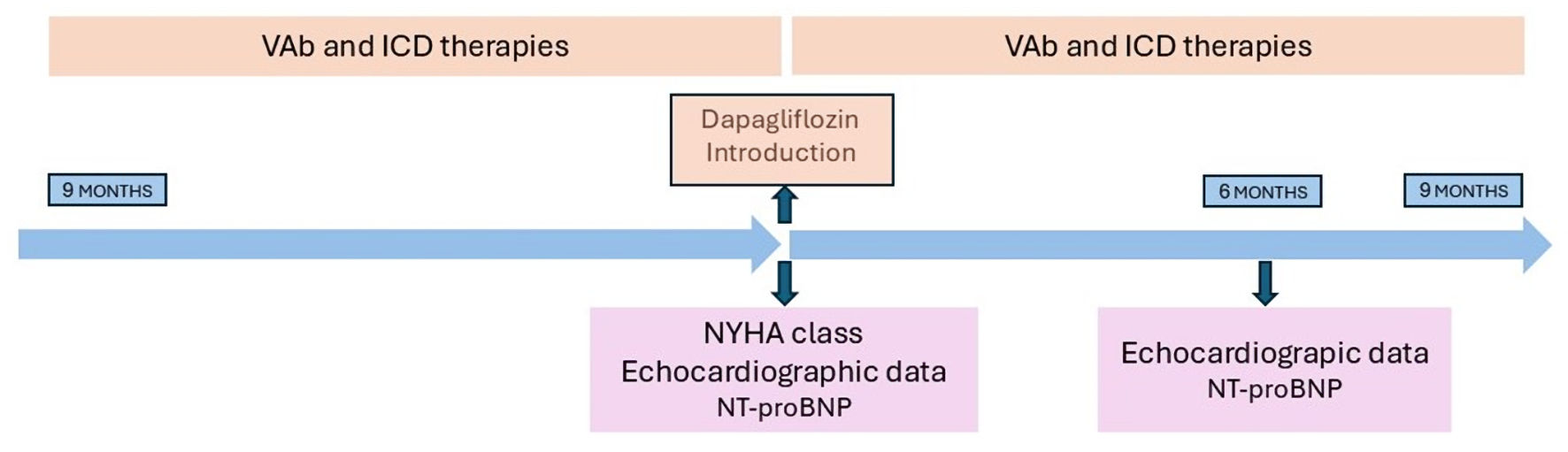 Click for large image | Figure 2. Observational study protocol main phases. ICD: implantable cardioverter defibrillator; VAb: ventricular arrhythmia burden; NYHA: New York Heart Association; NT-proBNP: N-terminal B-type natriuretic peptide. |
Arrhythmic events and ICD therapy
All of the devices were programmed in MADIT-RIT mode.
Ventricular arrhythmic burden data, including sustained ventricular tachycardia (sVT), non-sustained ventricular tachycardia (nsVT), ventricular fibrillation (VF), and total ventricular arrhythmias (VTA), together with specific ICD therapies (ICDt, appropriate ICD shocks and anti-tachycardia pacing (ATP)), were extrapolated from the analysis of the devices, considering the events present during the observation period pre- and post-introduction of dapagliflozin. Overall, 41% of patients had a remote monitoring device. A respectable number of patients were followed by way of remote monitoring, which is now recognized as a useful and reliable monitoring tool, especially in the population with HF [23, 24].
The observation period comprised 268 ± 42 days before and 272 ± 57 days after dapagliflozin introduction. Courtain et al [5] investigated the effect of dapagliflozin on the number of severe ventricular arrhythmias, resuscitated cardiac arrests, and sudden deaths reported during the follow-up to the DAPA-HF study. The separation of the Kaplan-Meier curves with respect to the composite endpoints is clearer by the ninth month, leading to the hypothesis of an indirect role of inverse remodeling. For this reason, a follow-up period for the evaluation of ventricular arrhythmic events of about 9 months was considered. Arrhythmic events and ICDt were identified as events per patient per month (Fig. 2).
Statistical analysis
The statistical analysis was conducted using the IBM SPSS Statistics v.16 software. Continuous variables are presented as the mean ± standard deviation. Qualitative variables are presented as absolute and percentage frequency. The data were normally distributed, as assessed by Shapiro-Wilk’s test of normality (P > 0.05). A kurtosis and skewness test was carried out with the application of the Student’s t-test for paired data and two-way mixed analysis of variance (ANOVA) to determine whether there are differences between two independent groups over time. Statistical significance was established at an alpha = 0.05.
| Results | ▴Top |
Population characteristics
A total of 298 outpatients with chronic HF were screened based on the previously reported inclusion and exclusion criteria. Of these, 117 patients met the eligibility criteria for enrolment in the study. Overall, 69.2% of the patients were male (81/117), while 30.8% were female (36/117), with a mean age of 70.3 ± 11.4 years. Based on reported symptoms, the patients were stratified according to NYHA functional classes, with 62 patients (52.4%) in the NYHA I/II classes and 55 patients (47.3%) in the NYHA III/IV classes. Etiology and comorbidity were investigated for all of the patients. Specifically, 50 patients (43%) were found to have ischemic cardiomyopathy (ICM), 67 patients (57%) had non-ischemic cardiomyopathy (NICM), 79 patients (67%) had hypertension (HTN), 29 patients (25%) had chronic kidney disease (CKD), 35 patients (30%) had diabetes mellitus (DM), 21 patients (18%) had chronic obstructive pulmonary disease (COPD), and 38 patients (32%) presented with a history of atrial fibrillation (AF). HTN was the most common condition in the sample (67%) with a higher incidence in the male sex. Similar incidence was observed in both sexes regarding DM (30%). The most common etiology was NICM (57%), which was more evident in women (61%), followed by ICM (43%), which was more evident in men (44%). A greater presence of chronic atrial fibrillation (28%) and consequent therapy with “Ablate and Pace” (11%) was also observed in women. Overall, 30 patients (25.6%) had a dual-chamber ICD (ICD-dc), 65 patients (55.5%) had a biventricular ICD (ICD-biv), and 22 patients (19%) had a single-chamber ICD (ICD-sc). Ninety-five defibrillators (80%) were implanted for the primary prevention of sudden cardiac death, and 12 defibrillators (10%) for secondary prevention. Regarding the distribution of pharmacological therapies, 111 patients (94.8%) were on beta blocker therapy, 71 patients (60.6%) were on angiotensin receptor/neprilysin inhibitor treatment (ARNI), 72 patients (61.5%) were on anti-aldosterone treatment (mineralocorticoid receptor antagonists (MRA)), 40 patients (34%) were on angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs), 82 patients (70%) were on furosemide, 10 patients (8.5%) were on digoxin, 30 patients (26%) were on amiodarone, 69 patients (58.4%) were on statin, 38 patients (32%) were on anticoagulants, and 71 patients (61%) were on antiplatelets. The baseline demographic, etiology, comorbidity, and pharmacotherapy history data are detailed in Table 1.
 Click to view | Table 1. Baseline Demographic, Etiology, Comorbidity, and Pharmacotherapy History Data |
Our analysis of the echocardiographic parameters indicates a mean LVEF of 25.9±2.3%, a mean E/E’ of 15.2 ± 2.3, a mean GLS of -12.6±0.9%, and a mean PASP of 37.9 ± 3.9 mm Hg. Patients with NYHA class III/IV had more impaired indices of systolic function and hemodynamic status than the patients with NYHA class I/II. Regarding the laboratory tests, a higher hematocrit value and a more compromised estimated glomerular filtration rate (eGFR) were observed in patients with NYHA class III/IV. The echocardiographic data and laboratory tests are detailed in Table 2.
 Click to view | Table 2. Echocardiographic Data and Laboratory Tests |
Changes in echocardiographic parameters and NT-proBNP
Sixty-nine patients (59%) had significant indices of reverse remodeling. The analysis of the population data shows a significant improvement during the observation period in mean LVEF (28.7±3.6% vs. 25.9±2.3%, P = 0.025), mean E/E’ (13.1 ± 1.1 vs. 15.2 ± 2.3, P = 0.035), and mean GLS (-14.7±1.2% vs. -12.6±0.9%, P = 0.03). There was no significant reduction in PASP (34.1 ± 3.2 mm Hg vs. 37.9 ± 3.9 mm Hg, P = 0.054). The improvement in the NT-proBNP parameter was also significant (1,136.1 ± 629.54 pg/L vs. 1,333.74 ± 564.3 pg/L, P = 0.02). The changes in the echocardiographic parameters and NT-proBNP are summarized in Table 3.
 Click to view | Table 3. Changes in the Echocardiographic Parameters and NT-proBNP |
Changes in arrhythmic events and ICDt
The analysis of ventricular arrhythmias showed a significant reduction in ventricular tachycardia (VT, 2.9 ± 1.8 after treatment vs. 4.5 ± 2.0 before treatment, per patient per month, P = 0.01), sVT (0.51 ± 0.77 after treatment vs. 1.2 ± 0.9 before treatment, per patient per month, P = 0.001), and nsVT (2.65 ± 1.58 after treatment vs. 3.84 ± 0.64 before treatment, per patient per month, P = 0.01) during the observation period. Concerning VF episodes, a non-significant trend for fewer events could be detected (0.00 ± 0.00 after treatment vs. 0.05 ± 0.02 before treatment, events per patient per month, P = 0.11).
The rate of all ICDt (ATP and ICD shock) decreased significantly during the observational period (0.49 ± 0.76 after treatment vs. 0.89 ± 1.38 before treatment, events per patient per month, P = 0.014). While there was only a non-significant trend toward a reduction in appropriate ICD shocks (0.00 ± 0.0 after treatment vs. 0.02 ± 0.03 before treatment, events per patient per month, P = not significant (NS)). The burden of appropriate ATPs was significantly reduced (0.57 ± 0.80 ATPs after treatment vs. 0.65 ± 0.91 ATPs before treatment, events per patient per month, P = 0.03). The arrhythmic events and ICD therapy, which were assessed in two distinct groups before and after dapagliflozin introduction, are detailed in Table 4.
 Click to view | Table 4. Arrhythmic Events and ICD Therapy Were Assessed in Two Distinct Groups Before and After Dapagliflozin (DAPA) Introduction |
Change in arrhythmic events and NYHA class
Patients with a more advanced functional class show a greater reduction in total ventricular events compared to patients with a better functional class. In the NYHA III/IV group, the number of VTA cases before treatment (events per patient per month) was 5.5 ± 1.8, while it was 2.2 ± 0.8 after treatment (P = 0.001). In the NYHA I/II group, the number of VTA cases before treatment (events per patient per month) was 4.5 ± 2.2 vs. 3.5 ± 2.1 after treatment (P = 0.02) (Fig. 3).
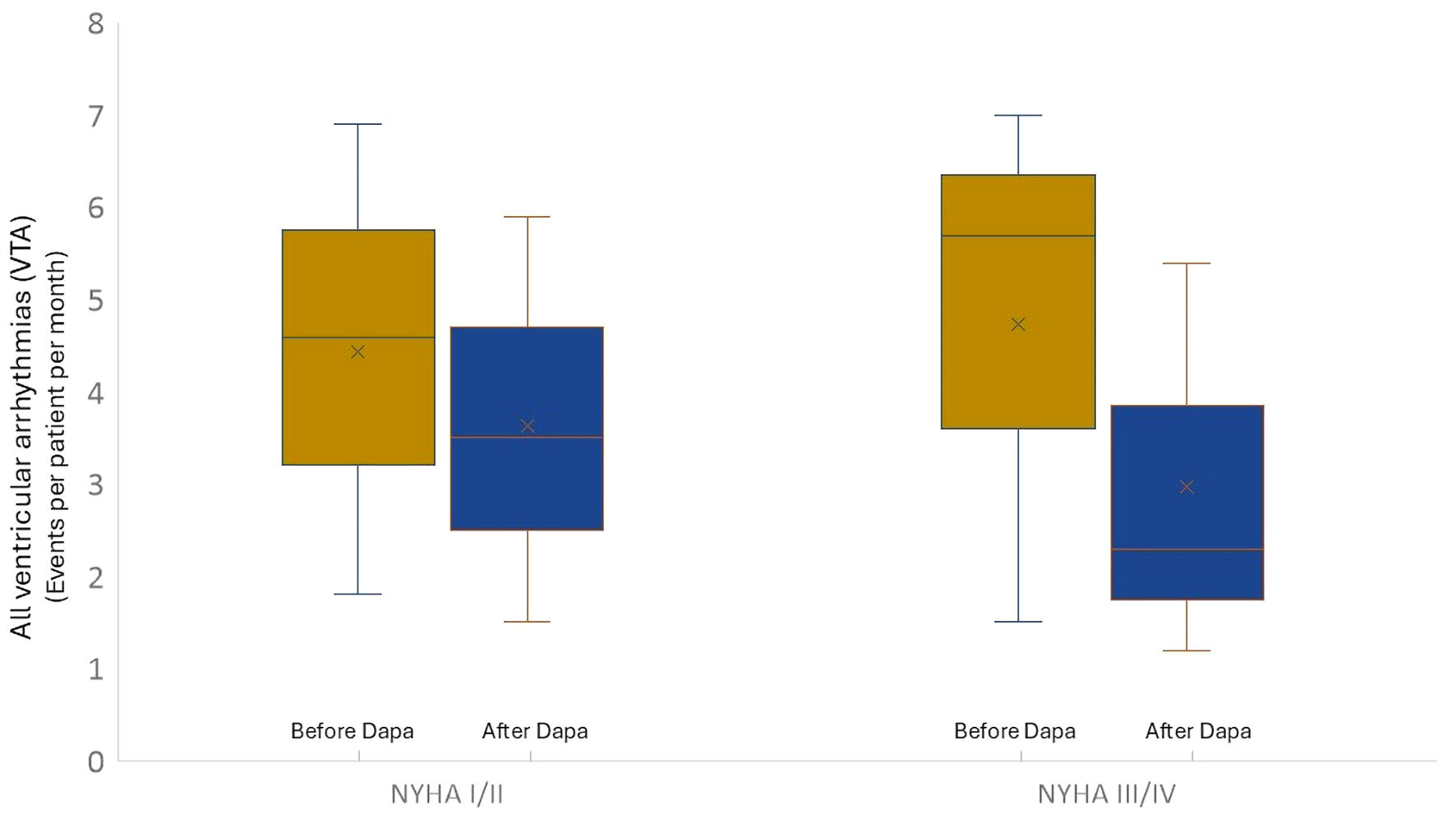 Click for large image | Figure 3. Trend of total ventricular arrhythmic events considering the basal NYHA functional class. Dapa: dapagliflozin; NYHA: New York Heart Association. |
The rate of appropriate ICDt (ATP and ICD shock) decreased significantly during the observational period in the NYHA III/IV group, from 0.66 ± 0.46 before treatment to 1.1 ± 1.08 after treatment (events per patient per month) (P = 0.02), but no significant reduction was observed in the NYHA I/II group, with an ICDt of 0.69 ± 0.33 before treatment vs. an ICDt after treatment of 0.52 ± 0.41 (events per patient per month, P = 0.14). Arrhythmic events and ICD therapy, which were assessed in two distinct groups according to the basal functional class (NYHA I/II and NYHA III/IV), are detailed in Table 5.
 Click to view | Table 5. Arrhythmic Events and ICD Therapy That Were Assessed in Two Distinct Groups According to the Basal Functional Class (NYHA I/II and NYHA III/IV) |
According to NYHA class, the variation of VTA in two independent groups was evaluated simultaneously by means of a two-way ANOVA test. There was a statistically significant interaction with large effect size between VTA improvement and NYHA class (F(1,115) = 142.25, P< 0.0001, partial η2 = 0.553) (Fig. 4).
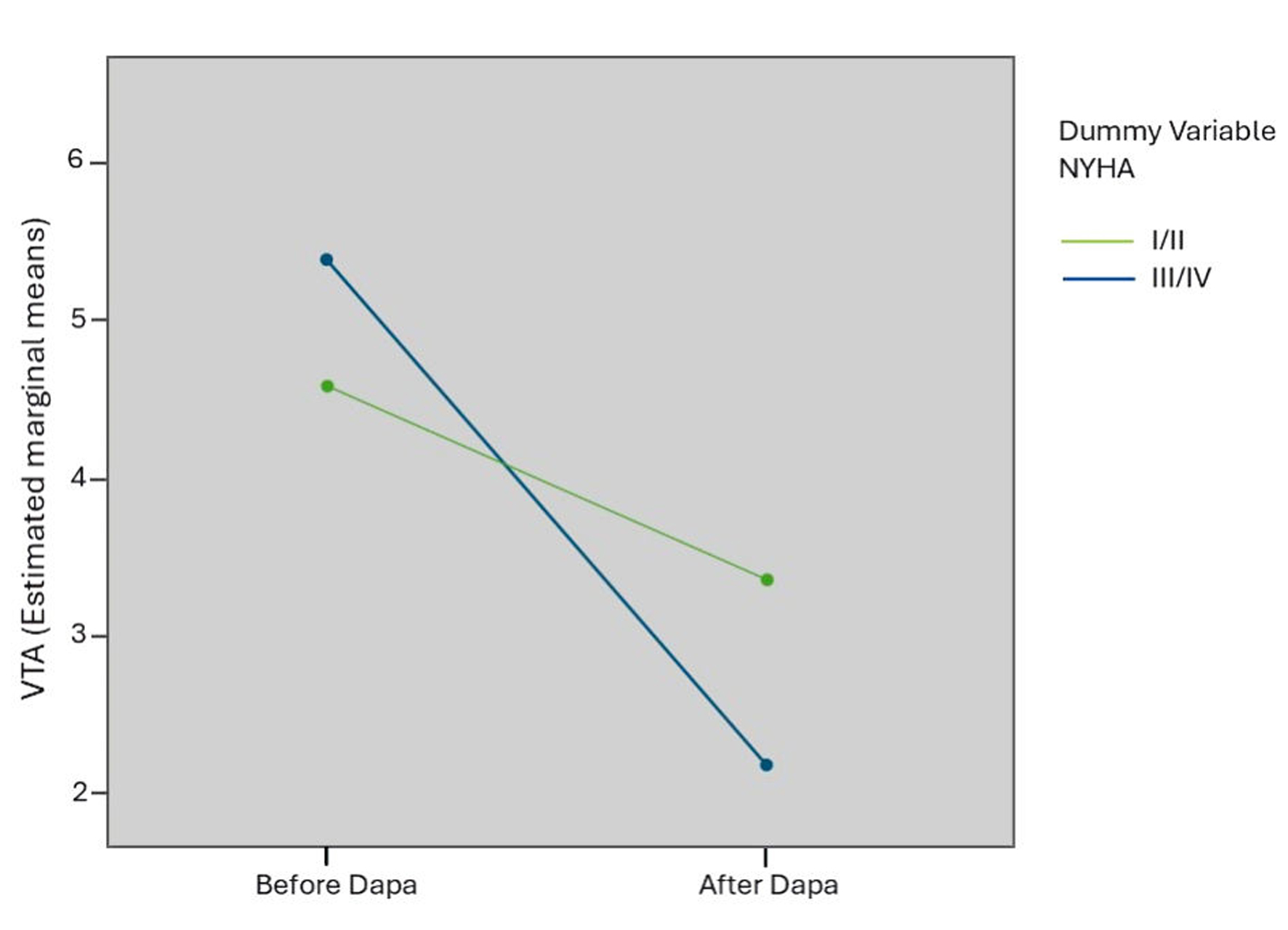 Click for large image | Figure 4. Interaction chart between VTA improvement and NYHA class, obtained by a two-way ANOVA test. VTA: total ventricular arrhythmias; NYHA: New York Heart Association; ANOVA: analysis of variance; Dapa: dapagliflozin. |
Changes in arrhythmic events and ventricular reverse remodeling
Upon dividing the population according to ventricular reverse remodeling, a significant difference in the reduction in the number of ventricular arrhythmic events is observed.
Patients with significant reverse remodeling (ΔLVEF > 15%, n = 50) showed a greater reduction in the total number of ventricular events from 5.1 ± 1.6 before treatment to 2.5 ± 1.1 after treatment (events per patient per months, P = 0.01), but no significant reduction is observed in the patients without significant reverse remodeling (ΔLVEF < 15%, n = 67, 4.9 ± 2.1 before treatment vs. 4.5 ± 2.1 after treatment (events per patient per month), P = NS) (Fig. 5).
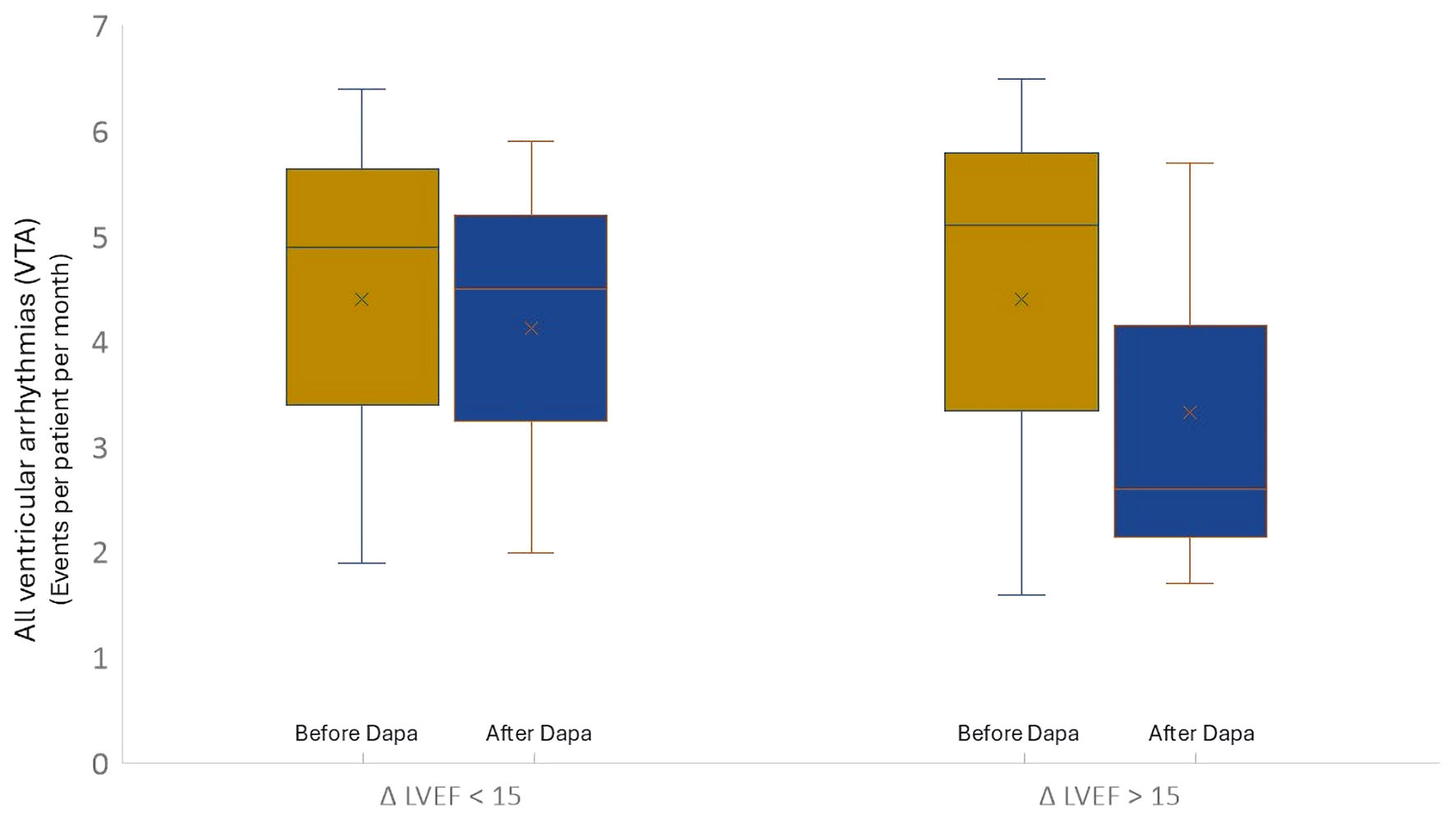 Click for large image | Figure 5. Trend of total ventricular arrhythmic events considering the ventricular reverse remodeling. ΔLVEF < 15%: patients without ventricular reverse remodeling; ΔLVEF > 15%: patients with ventricular reverse remodeling). Dapa: dapagliflozin; NYHA: New York Heart Association; LVEF: left ventricular ejection fraction. |
The rate of appropriate ICDt (ATP and ICD shock) decreased significantly during the observational period in patients with significant reverse remodeling (ΔLVEF > 15%, 1.0 ± 1.1 before treatment vs. 0.77 ± 0.42 after treatment (events per patient per month), P = 0.02), but no significant reduction was observed in the patients without reverse remodeling (ΔLVEF < 15%, 0.58 ± 0.31 before treatment vs. 0.53 ± 0.40 after treatment (events per patient per month), P = NS). Arrhythmic events and ICD therapy, which were assessed in two groups divided by ventricular reverse remodeling, are detailed in Table 6.
 Click to view | Table 6. Arrhythmic Events and ICD Therapy That Were Assessed in Two Groups Divided by Ventricular Reverse Remodeling |
The variation of VTA in two independent groups according to ventricular reverse remodeling was evaluated simultaneously by means of a two-way ANOVA test. There was a statistically significant interaction with a large effect size between VTA improvement and ΔLVEF (F(1,115) = 107,678, P < 0.0001, partial η2 = 0.484) (Fig. 6).
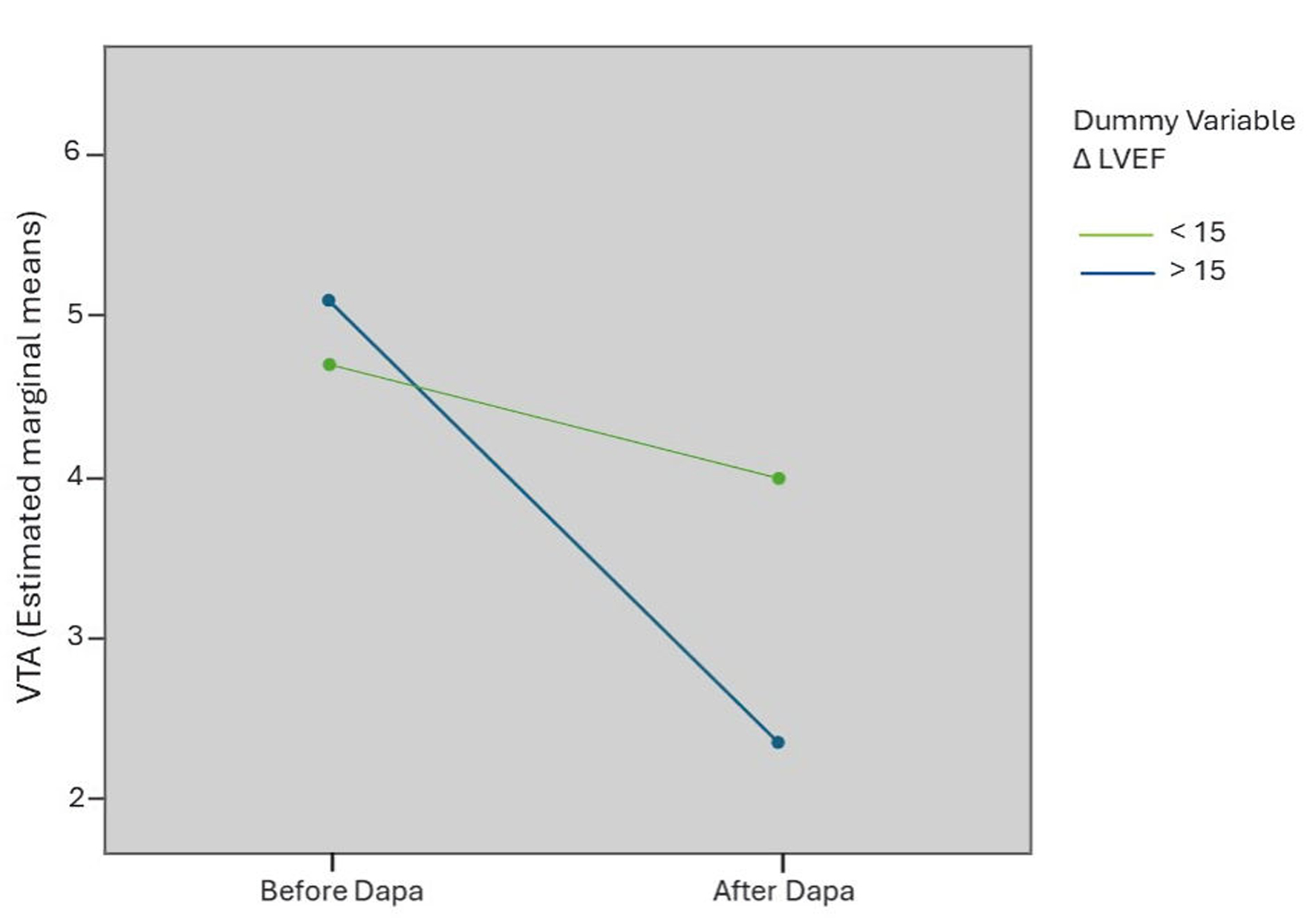 Click for large image | Figure 6. Interaction chart between VTA improvement and ventricular reverse remodeling, obtained by a two-way ANOVA test. VTA: total ventricular arrhythmias; LVEF: left ventricular ejection fraction; ANOVA: analysis of variance; Dapa: dapagliflozin. |
Change in ventricular arrhythmic events, subgroup analysis
We studied the changes in VTA and ICDt in various subgroups and evaluated the interaction effect of the selected variables.
The subgroups studied included: diabetic/non-diabetic patients, male/female, guideline- directed medical therapy (GDMT)/suboptimal-GDMT, amiodarone/no amiodarone, ICM/NICM (Table 7).
 Click to view | Table 7. Change in the Ventricular Arrhythmic Events in Various Subgroups |
GDMT meant the presence of the four pillars of optimal medical therapy according to the guidelines (70/117). The suboptimal-GDMT subgroup (47/117) provided for the absence of at least one of the four pillars (MRA in 38.5% of cases). A significant reduction in ventricular arrhythmic events was observed in all subgroups. A two-way ANOVA test shows a small effect size for the variables studied, except GDMT, which presented a large effect size (P = 0.03, η2 = 0.184) (Table 7).
Regarding the changes in ICDt, a significant reduction was observed after the subdivision of the subgroups according to the selected variables, but it was not possible to perform an interaction analysis for the small number of events.
| Discussion | ▴Top |
SGLT2is have been shown to be effective in reducing the occurrence of death and hospitalization in HF patients [1-4]. Ventricular arrhythmias are common in patients with HF, especially of systolic genesis. Although several studies have shown that the use of SGLT2i is associated with a reduction in the number of atrial and ventricular arrhythmic events [5, 6], the burden of cardiac arrhythmias is still a matter of debate, especially in the field of ventricular burden. Several potential direct antiarrhythmic actions related to SGLT2 inhibitors have been hypothesized [12-19]. The most intuitive indirect antiarrhythmic effect is the improvement of hemodynamic status and the structural remodeling induced by the treatment. Therefore, in our study, we tried to highlight whether the functional class, i.e., the pre-treatment clinical status, could influence improvements in ventricular events and, in the same way, analyze whether treatment-induced remodeling was related to the trend of ventricular arrhythmic events and ICDt.
Arrhythmic events and NYHA class
Upon analyzing the data of our study, a significant reduction in the number of ventricular events and ATP therapies is observed in the short-medium term. Dividing the population according to the basal NYHA functional class, there was a more significant improvement in VAb in the group of patients with a more compromised functional class. To confirm the hypothesis of a statistically significant interaction between VTA variation and basal functional class, a two-way ANOVA test was used, which showed an interaction with a large effect size. This finding could be justified by the benefits of the treatment-related immediate volume depletion effect, which is more evident in patients with a worse functional class and with a greater hemodynamic overload. In support of this hypothesis, our data confirm that the group of patients with a more compromised functional class presents with echocardiographic and biochemical indices indicative of greater hemodynamic overload. Although in the DAPA-HF trial, the baseline functional class does not influence the study endpoints, a subsequent analysis of the DAPA-HF data [25] showed that clinical benefits in the short-medium term were observed in patients who were more symptomatic at baseline. This benefit of dapagliflozin is mostly observed in the first 4 months and then stabilizes in the following period. Various well-known pieces of evidence demonstrate the lack of short-term efficacy of SGLT2i on the hemodynamic parameters in low-risk patients. A previous study showed that the most stable patients did not experience a reduction in BNP after treatment with SGLT2i in the short-term period, while other studies have shown that there was no significant improvement in the ventricular filling indices or signs of diastolic function among more stable patients, compared to more clinically compromised patients [8, 26]. Some studies showed that the starting functional class modifies the result in terms of reverse remodeling in the short-medium term; this is more evident in patients with a more compromised clinical state [27, 28]. This evidence could support the hypothesis of the indirect antiarrhythmic action of SGLT2i influenced by short-term hemodynamic improvement.
Arrhythmic events and ventricular remodeling
In our work, we also evaluated the correlation between remodeling and the reduction in the occurrence of ventricular events. As is well known, the reversal of cardiac remodeling is associated with clinical improvement, a better quality of life, and a reduction in the risk of hospitalization and death from HF [29-31]. Cardiac remodeling is a complex process involving various pathophysiological pathways, including inflammation, oxidative stress, metabolic abnormalities, mitochondrial dysfunction, autophagy, and apoptosis, resulting in myocyte loss, cardiac hypertrophy, and interstitial fibrosis [32-34]. SGLT2is exhibit favorable hemodynamic and vascular effects mediated by several mechanisms that have been shown to reduce cardiac preload and afterload, and thus could mitigate left ventricular (LV) stretching and wall stress, leading to a reduction in the LV volume and an improvement in systolic function indices [7-11, 35-37]. In our study, upon dividing the population according to ventricular reverse remodeling, a significant difference in the reduction in the occurrence of ventricular arrhythmic events was observed. Patients with significant reverse remodeling show a greater reduction in total ventricular events, but no significant reduction is observed for the patients without significant reverse remodeling. To confirm the hypothesis of a statistically significant interaction between VTA variation and ventricular reverse remodeling, a two-way ANOVA test was used, which showed an interaction with a large effect size.
Arrhythmic events and GDMT
In all of the subgroups divided according to several variables, a significant reduction in arrhythmic ventricular events and ICD therapy was observed. By way of interaction analysis, we observed an interaction with a large effect size between optimized therapy and the benefit of dapagliflozin. Dapagliflozin produces a greater benefit in patients with optimized medical therapy compared to patients who did not undergo optimized therapy. This result could be explained by a hypothetical positive interaction between all four pillars used in HF, which justify the better benefit observed when used together.
Conclusions
In summary, in this observational study, dapagliflozin produces ventricular reverse remodeling and reduces the incidence of ventricular arrhythmias in patients with HFrEF. The benefit for ventricular arrhythmic events appears to be more significant in patients with a reduced basal functional capacity, related to the hemodynamic improvement obtained through the treatment, and in patients with significant reverse remodeling. Therefore, we can hypothesize that, although the pleiotropic effects of SGLT2is are varied and constantly evolving, a significant reduction in VAb at least in the short-medium term, is indirectly obtained by dapagliflozin treatment, inducing hemodynamic and structural improvements.
Study limitations
Our study had several limitations. First, the small size of the population examined certainly affects the statistical evaluation of the comparison between means and, therefore, it would be desirable to evaluate a larger population to make the correlation analysis reliable. The exclusion of patients with various conditions of instability during the observation reduced the sample size of the population. Secondly, ventricular events were evaluated for a brief period before and after treatment, meaning that, for some forms of arrhythmia, such as VF, the number is so small that it has no statistically significant value. As a result, our findings should be regarded as hypothesis-generating and, therefore, they require confirmation.
Acknowledgments
We thank the Department of Life, Health and Environmental Sciences, University of L’Aquila, Italy and Cardiomed Medical Center, Maglie, Italy.
Financial Disclosure
No funding was received.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Informed Consent
All patients provided written informed consent.
Author Contributions
Conceptualization, Gabriele D.M. and Z.P.; methodology, Gabriele D.M., Z.P., S.L. and F.B.; validation, S.R., L.S.; formal analysis, Z.P. and Gabriele D.M.; original draft preparation, Gabriele D.M., Z.P., S.L., F.B. and M.C.; writing - review and editing, Z.P. and Giuseppe D.M.; supervision, A.G.R, M.N., A.S., P.P., G.C., M.C. S.M., L.F., and Giuseppe D.M. All authors have read and agreed to the published version of the manuscript
Data Availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
VAb: ventricular arrhythmia burden; HF: heart failure; ICD: implantable cardioverter defibrillator; HFrEF: heart failure with reduced ejection fraction; NYHA: New York Heart Association; CRT: cardiac resynchronization therapy; EACVI: European Association of Cardiovascular Imaging; sVT: sustained ventricular tachycardia; nsVT: non-sustained ventricular tachycardia; VF: ventricular fibrillation; VTA: total ventricular arrhythmias; ATP: anti-tachycardia pacing; ICDt: ICD therapies; HTN: hypertension; ICM: ischemic cardiomyopathy; NICM: non-ischemic cardiomyopathy; DM: diabetes mellitus; AFib-c: chronic atrial fibrillation; AFib-p: paroxysmal atrial fibrillation; ICD-dc: dual-chamber implantable cardioverter defibrillator; ICD-biv: biventricular implantable cardioverter defibrillator; ICD-sc: single-chamber cardioverter defibrillator; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; ACEI: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; MRA: mineralocorticoid receptor antagonists; APT: antiplatelet therapy; LVEF: left ventricular ejection fraction; GLS: global longitudinal strain; E/E’: ventricular filling index; PASP: pulmonary arterial systolic pressure; eGFR: estimated glomerular filtration rate; GDMT: guideline-directed medical therapy
| References | ▴Top |
- McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008.
doi pubmed - Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424.
doi pubmed - Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396(10254):819-829.
doi pubmed - Peikert A, Martinez FA, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, Jhund PS, et al. Efficacy and safety of dapagliflozin in heart failure with mildly reduced or preserved ejection fraction according to age: the DELIVER trial. Circ Heart Fail. 2022;15(10):e010080.
doi pubmed - Curtain JP, Docherty KF, Jhund PS, Petrie MC, Inzucchi SE, Kober L, Kosiborod MN, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. 2021;42(36):3727-3738.
doi pubmed - Younis A, Arous T, Klempfner R, Kharsa A, McNitt S, Schleede S, Polonski B, et al. Effect of sodium glucose cotransporter 2 inhibitors on atrial tachy-arrhythmia burden in patients with cardiac implantable electronic devices. J Cardiovasc Electrophysiol. 2023;34(8):1595-1604.
doi pubmed - Omar M, Jensen J, Frederiksen PH, Kistorp C, Videbaek L, Poulsen MK, Moller S, et al. Effect of empagliflozin on hemodynamics in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2020;76(23):2740-2751.
doi pubmed - Jensen J, Omar M, Kistorp C, Poulsen MK, Tuxen C, Gustafsson I, Kober L, et al. Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: A double-blinded, randomized, and placebo-controlled trial. Am Heart J. 2020;228:47-56.
doi pubmed - Omar M, Jensen J, Ali M, Frederiksen PH, Kistorp C, Videbaek L, Poulsen MK, et al. Associations of empagliflozin with left ventricular volumes, mass, and function in patients with heart failure and reduced ejection fraction: a substudy of the empire HF randomized clinical trial. JAMA Cardiol. 2021;6(7):836-840.
doi pubmed - Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, Macaluso F, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77(3):243-255.
doi pubmed - Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. 2021;143(6):516-525.
doi pubmed - Shimizu W, Kubota Y, Hoshika Y, Mozawa K, Tara S, Tokita Y, Yodogawa K, et al. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020;19(1):148.
doi pubmed - Philippaert K, Kalyaanamoorthy S, Fatehi M, Long W, Soni S, Byrne NJ, Barr A, et al. Cardiac late sodium channel current is a molecular target for the sodium/glucose cotransporter 2 inhibitor empagliflozin. Circulation. 2021;143(22):2188-2204.
doi pubmed - Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, Jancev M, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia. 2018;61(3):722-726.
doi pubmed - Chung YJ, Park KC, Tokar S, Eykyn TR, Fuller W, Pavlovic D, Swietach P, et al. SGLT2 inhibitors and the cardiac Na+/H+ exchanger-1: the plot thickens. Cardiovasc Res. 2021;117(14):2702-2704.
doi pubmed - Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, et al. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18(1):165.
doi pubmed - Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65(5):1190-1195.
doi pubmed - Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a State-of-the-Art review. JACC Basic Transl Sci. 2020;5(6):632-644.
doi pubmed - Ekanayake P, Hupfeld C, Mudaliar S. Sodium-glucose cotransporter type 2 (SGLT-2) inhibitors and ketogenesis: the good and the bad. Curr Diab Rep. 2020;20(12):74.
doi pubmed - Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233-270.
doi pubmed - Pascual-Figal DA, Zamorano JL, Domingo M, Morillas H, Nunez J, Cobo Marcos M, Riquelme-Perez A, et al. Impact of dapagliflozin on cardiac remodelling in patients with chronic heart failure: The DAPA-MODA study. Eur J Heart Fail. 2023;25(8):1352-1360.
doi pubmed - Xanthopoulos A, Katsiadas N, Skoularigkis S, Magouliotis DE, Skopeliti N, Patsilinakos S, Briasoulis A, et al. Association between dapagliflozin, cardiac biomarkers and cardiac remodeling in patients with diabetes mellitus and heart failure. Life (Basel). 2023;13(8):1778.
doi pubmed - de Ruvo E, Sciarra L, Martino AM, Rebecchi M, Iulianella RV, Sebastiani F, Fagagnini A, et al. A prospective comparison of remote monitoring systems in implantable cardiac defibrillators: potential effects of frequency of transmissions. J Interv Card Electrophysiol. 2016;45(1):81-90.
doi pubmed - Sciarra L, Nesti M, Palama Z, Marazzato J, Bagliani G, Leonelli FM, De Ponti R. Arrhythmias in patients with implantable devices. Card Electrophysiol Clin. 2019;11(2):363-373.
doi pubmed - Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation. 2020;141(2):90-99.
doi pubmed - Soga F, Tanaka H, Tatsumi K, Mochizuki Y, Sano H, Toki H, Matsumoto K, et al. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol. 2018;17(1):132.
doi pubmed - Hundertmark MJ, Adler A, Antoniades C, Coleman R, Griffin JL, Holman RR, Lamlum H, et al. Assessment of cardiac energy metabolism, function, and physiology in patients with heart failure taking empagliflozin: the randomized, controlled EMPA-VISION trial. Circulation. 2023;147(22):1654-1669.
doi pubmed - Requena-Ibanez JA, Santos-Gallego CG, Rodriguez-Cordero A, Vargas-Delgado AP, Mancini D, Sartori S, Atallah-Lajam F, et al. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM study. JACC Heart Fail. 2021;9(8):578-589.
doi pubmed - Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL, Jr. Imaging, biomarker, and clinical predictors of cardiac remodeling in heart failure with reduced ejection fraction. JACC Heart Fail. 2019;7(9):782-794.
doi pubmed - Bhatt AS, Ambrosy AP, Velazquez EJ. Adverse remodeling and reverse remodeling after myocardial infarction. Curr Cardiol Rep. 2017;19(8):71.
doi pubmed - Jaiswal A, Nguyen VQ, Carry BJ, le Jemtel TH. Pharmacologic and endovascular reversal of left ventricular remodeling. J Card Fail. 2016;22(10):829-839.
doi pubmed - Schirone L, Forte M, Palmerio S, Yee D, Nocella C, Angelini F, Pagano F, et al. A review of the molecular mechanisms underlying the development and progression of cardiac remodeling. Oxid Med Cell Longev. 2017;2017:3920195.
doi pubmed - Frantz S, Hundertmark MJ, Schulz-Menger J, Bengel FM, Bauersachs J. Left ventricular remodelling post-myocardial infarction: pathophysiology, imaging, and novel therapies. Eur Heart J. 2022;43(27):2549-2561.
doi pubmed - Salah HM, Verma S, Santos-Gallego CG, Bhatt AS, Vaduganathan M, Khan MS, Lopes RD, et al. Sodium-glucose cotransporter 2 inhibitors and cardiac remodeling. J Cardiovasc Transl Res. 2022;15(5):944-956.
doi pubmed - Marketou M, Kontaraki J, Maragkoudakis S, Danelatos C, Papadaki S, Zervakis S, Plevritaki A, et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiac structural and electrical remodeling: from myocardial cytology to cardiodiabetology. Curr Vasc Pharmacol. 2022;20(2):178-188.
doi pubmed - Seferovic PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, Bauersachs J, et al. Sodium-glucose co-transporter 2 inhibitors in heart failure: beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22(9):1495-1503.
doi pubmed - Correale M, Lamacchia O, Ciccarelli M, Dattilo G, Tricarico L, Brunetti ND. Vascular and metabolic effects of SGLT2i and GLP-1 in heart failure patients. Heart Fail Rev. 2023;28(3):733-744.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.