| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 15, Number 6, December 2024, pages 439-452
Safety and Efficacy of BioMime Sirolimus-Eluting Stent System in All-Comers Real-World Population With Coronary Artery Stenosis: MILES Global Registry
Martin Hudeca, x , Myung Ho Jeongb, Ramiro Trilloc, Alexander J.J. Ijsselmuidend, Hyeon-Cheol Gwone, In Ho Chaef, Yi-Chih Wangg, Jose Maques de Costah, Min-Ji Charngi, Oteh Maskonj, Jose Moreu Burgosk, Gnanamoorthy Mayurathanl, Hristo Mateevm, Antonio Serran, Bela Merkelyo, Rita Calep, Shinn-Jang Hwangq, Guang-Yuan Marr, Samih Lawands, Andriy Khokhlovt, Beatriz Vaqueizo Montillau, Mariano Valdesv, Mohammad Sadeghianw
aDepartment of Acute Cardiology, Middle-Slovak Institute of Cardiovascular Diseases (SUSCCH), Banska Bystrica, Slovakia
bDepartment of Cardiology, Chonnam National University, Gwangju, Korea
cDepartment of Cardiology, Complexo Hospitalario Universitario de Santiago de Compostela, Spain and Centro de Investigacion Biomedica en Red Enfermedades Cardiovasculares CIBER-CV, Madrid, Spain
dDepartment of Interventional Cardiology, Albert Schweitzer Hospital, The Netherlands
eDepartment of Cardiology, Samsung Medical Centre, Seoul, Korea
fDepartment of Internal Medicine, Bundang Seoul National University Hospital, Seongnam, Korea
gDepartment of Internal Medicine, National Taiwan University Hospital, Taiwan, Republic of China
hHospital Santa Maria, Lisbon, Portugal
iDivision of Cardiology, Shin Kong Wu Ho-Su Memorial Hospital, Taiwan, Republic of China
jDepartment of Medicine (Cardiology Unit), Universiti Kebangsaan Malaysia Medical Centre, Cheras, Malaysia
kDepartment of Cardiology, Hospital Virgen de la Salud, Toledo, Spain
lDepartment of Cardiology, Teaching Hospital Kandy, Sri Lanka
mDepartment of Interventional Cardiology, National Heart Hospital, Sofia, Bulgaria
nDepartment of Cardiology, Hospital de Sant Pau, Barcelona, Spain
oDepartment of Cardiology, University of Semmelweis, Budapest, Hungary
pDepartment of Cardiology, Hospital Garcia Orta, Almada, Portugal
qTaipei Medical University Hospital (TMUH), Taiwan, Republic of China
rDepartment of Critical Care Medicine, Veteran General Hospital (KVGH), Taiwan, Republic of China
sCardiovascular Department, Dallah Hospital, Riyadh, Saudi Arabia
tHeart Institute of The Ministry of Healthcare of Ukraine, Kyiv, Ukraine
uDepartment of Cardiology, Hospital Del Mar, Barcelona, Spain
vDepartment of Cardiology, Hospital Universitario Virgen de la Arrixaca, Murcia, Spain
wDepartment of Forensic Medicine, Imam Khomeini Hospital, Tehran, Iran
xCorresponding Author: Martin Hudec, Middle-Slovak Institute of Cardiovascular Diseases (SUSCCH), Banska Bystrica, Slovakia
Manuscript submitted September 2, 2024, accepted October 30, 2024, published online December 3, 2024
Short title: BioMime SES: MILES Global Registry
doi: https://doi.org/10.14740/cr1724
| Abstract | ▴Top |
Background: This study evaluated the safety and efficacy of BioMime sirolimus-eluting stent (SES) system, with an ultra-low strut thickness (65 µm), in real-world all-comers population with coronary artery stenosis (CAD).
Methods: This was a post-marketing, multicenter, single-arm, observational clinical registry among patients undergoing intervention for CAD. Patients were clinically followed up at 1, 9, 12, and 24 months after the index percutaneous coronary intervention. Four major indications, namely long stents of > 30 mm, stents with diameters of 4 and 4.5 mm, bifurcation subgroup, and chronic total occlusion (CTO) were evaluated as pre-specified subsets.
Results: A total of 771 patients (1,079 treated lesions) from 23 sites were included in this study. The mean length and diameter of the implanted stents were 25.57 ± 9.35 mm and 3.00 ± 0.44 mm, respectively. The mean minimum lumen diameter before and after the procedure was 1.00 ± 1.69 mm and 2.96 ± 1.35 mm, respectively. The cumulative rates of major adverse cardiovascular events (MACEs) and stent thrombosis (ST) at 1, 9, 12, and 24 months were 1.05%, 3.13%, 4.04%, 5.64% and 0%, 0.13%, 0.28%, 0.28%, respectively. In a subset with > 30 mm long stents, the cumulative rate of MACEs was 0.4%, 4.6%, 5.12%, and 7.01% at 1, 9, 12, and 24 months, respectively. The corresponding rates of ST were 0%, 0.42%, 0.43%, and 0.44%, indicating constant rate of ST after 9 months. In a subset of 4 and 4.5 mm diameter stents, the cumulative rate of MACEs was high (0%, 6.25%, 6.25%, and 10.41%) at 1, 9, 12, and 24 months, respectively. However, there was no case of ST until 24 months. In patients with bifurcation lesions, the cumulative rates of MACEs and ST were 2.46%, 6.32%, 11.53%, 16.21% and 0%, 1.27%, 1.28%, 1.35% at 1, 9, 12, and 24 months follow-up. In patients with chronic total occlusion, the cumulative rates of MACEs and ST were 0.79%, 5.04%, 6.83%, 7.07% and 0%, 0.84%, 0.85%, 0.88% at 1, 9, 12, and 24 months, respectively, indicating constant rate of ST after 9 months.
Conclusions: The BioMime SES demonstrated good safety and efficacy outcomes at 24-month follow-up, with low rates of MACEs and ST in patients with CAD in the real-world setting.
Keywords: BioMime SES; Bifurcation lesions; Chronic total occlusion; Coronary artery disease; Drug-eluting stent; Stent thrombosis
| Introduction | ▴Top |
Drug-eluting stent (DES) implantation is a well-established and widely accepted treatment for coronary artery disease (CAD) [1]. Nevertheless, despite technological advances, it is associated with a small but significant risk of stent thrombosis (ST) and in-stent restenosis (ISR) [1]. ST is associated with high mortality and morbidity [2], while ISR (diameter stenosis > 50% within the stented segment) remains the most common cause of stent failure, accounting for 10-20% of all percutaneous coronary intervention (PCI) procedures [3]. The newer-generation DESs aim to address the limitations observed in the earlier-generation DESs, focusing on all three components: the stent backbone, the anti-proliferative agent, and the drug carrier [1]. The first-generation DES was designed using stainless steel platforms (iron, nickel, and chromium) with a strut thickness of 130 - 150 µm. In contrast, the newer-generation DES employs cobalt chromium (CoCr) and platinum chromium (PtCr), to achieve thinner struts (< 100 µm) that reduce shear stress, promote faster and complete endothelial strut coverage, and maintain adequate radial strength [4]. Notably, thinner struts are associated with lower inflammation at the stented arterial segment, reduced thrombogenicity, less neointimal hyperplasia, and a lower risk of clinically-driven target lesion revascularization (TLR) and target vessel revascularization (TVR) [5].
The sirolimus-eluting stents (SESs) have consistently shown superior anti-restenotic efficacy over paclitaxel-eluting stents in the first-generation DESs. As a result, the newer-generation DESs predominantly use the -limus family of drugs [1, 6]. To minimize the inflammatory responses triggered by the persistence of polymer coating after drug release, contemporary DESs use biocompatible durable fluorinated or biodegradable polymers (made of lactic or glycolic acids) that fully resorb through hydrolysis after the drug is released [7].
The BioMime SES utilizes the NexGen™ CoCr coronary stent system to minimize intra-arterial injury, with an ultra-low strut thickness (65 µm), that maintains radial strength across all dimensions. Its hybrid cell design structure (open cell configurations in the center and closed cells at the edges) ensures minimal edge injury and complete wall apposition. Additionally, the stent delivery system minimizes arterial injury owing to carefully constructed semi-compliant rapid exchange balloon catheter shoulders. The drug employed is sirolimus, which is an ideal choice considering its action on the common final pathway of cell division cycle without exceptional risk of necrosis induction. The stent’s thin 2 µm stable coating utilizes non-inflammatory biodegradable polymers, namely poly-L-lactic acid (PLLA) and poly lactic-co-glycolic acid (PLGA) [8, 9].
While pre-clinical and clinical studies have demonstrated the safety and efficacy of BioMime SES [10-13], post-marketing surveillance remains essential to evaluate its “real-world” clinical performance in interventional clinical practice. Therefore, we conducted this study to assess the safety and efficacy of the BioMime SES system in real-world all-comers population with coronary artery stenosis seen in routine clinical practice.
| Materials and Methods | ▴Top |
Study design and patient population
The MILES Global Registry is a post-marketing, multicenter, single-arm, observational clinical registry in real-world all-comers population with coronary artery stenosis across 23 sites worldwide. The key inclusion criteria were males and females aged > 18 years, ability and willingness to provide voluntary written informed consent to participate in this clinical investigation. PCI and implantation of BioMime DES were performed as a part of treatment of CAD, without any further indication for emergent coronary artery bypass graft (CABG) surgery. The key exclusion criteria were: ineligibility for a PCI or need for urgent/planned elective CABG; use of stents other than BioMime; history of internal bleeding, planned surgery or any similar reason that restricted administration of dual antiplatelet therapy; > grade III renal insufficiency (creatinine > 160 µmol/L); unprotected left main artery lesion; life expectancy < 2 years. The detailed eligibility criteria are given in Supplementary Material 1 (cr.elmerpub.com).
The study was approved by the local ethics committee of all the participating centers and was performed per the principles stated in the Declaration of Helsinki.
The study’s design considered four major complex indications, namely long stents of > 30 mm, stents with diameters of 4 and 4.5 mm, bifurcation subgroup, and chronic total occlusion (CTO) were evaluated as pre-specified subsets. The subset assessment was performed based on the number of patients in each subgroup. Notably, lesion coverage with a single stent was encouraged where possible including in patients with long lesions (> 30 mm). When two or more adjacent stents were required, care was taken to achieve a 1 - 2 mm overlap and avoid short gaps between stents. Post-dilatation was strongly encouraged, ensuring that the post-dilatation balloon remained within the stented segment. In bifurcation lesions, single or two stent strategies were allowed at the operator’s discretion. If two stents were used, both had to be BioMime SESs. The CTO subgroup was considered for analysis if at least 10 patients (5% of the overall population) were available. Patients were clinically followed up at 1, 9, 12, and 24 months after the index PCI.
Study endpoints
The primary efficacy endpoint was rate of target vessel failure (TVF) at 9 months. It was defined as frequency of hierarchical, cumulative composite of cardiac death, myocardial infarction (MI) not clearly attributed to any other vessel, and TVR at 9 months. The primary safety endpoint was rate of sub-acute definite or probable ST as defined by the Academic Research Consortium (ARC) while on dual antiplatelet therapy.
Secondary endpoints included cumulative TVF at 1, 9, 12, and 24 months, TLR at 1, 9, 12, and 24 months, and major adverse cardiovascular events (MACEs) at 1, 9, 12, and 24 months. MACE was defined as an event of cardiac death, MI, or TVR. ST was defined as thrombosis within the stent area and 5 mm margin on either side from the edges of the stent. Frequency of ST was evaluated as acute (0 - 48 h after stent implant), late (1 month to 1 year after stent implant), and very late (beyond 1 year after stent implant) according to the ARC definitions: definite, probable, and possible, at all follow-up visits.
Procedure and post-PCI therapy
The patients were treated for CAD as per the standard guidelines and practice [14]. Only patients who received BioMime SESs, as indicated, were considered for recruitment. Pre-dilatation and post-dilation were optional and was at the discretion of the operator. The choice of vascular access for PCI (e.g., femoral or radial) was left to the operator’s judgment. A minimum of 1 year of dual antiplatelet therapy (aspirin and P2Y12 blocker) with indefinite aspirin or P2Y12 monotherapy was recommended. Electrocardiogram (ECG) and cardiac enzymes were evaluated post-procedure. Adverse events were recorded from randomization until the last subject’s last study visit or follow-up.
Sample size calculation
To estimate the sample size, the performance goal was determined by meta-analysis of the real-world registries: e-Cypher, Endeavour V, Spirit V, e-BioMatrix, Xience Prime Registry, etc. The performance goal was taken as comparator p that was 9.2% (0.092) of TVF at 9 months. For a proportion-based calculation by one-sample t-test on Minitab software, we tested a sample size of 120 for the desired confidence interval. The new comb-Wilson method yielded a 95% confidence interval between 92% and 90% (mean difference 0.019) with 98% power (β = 0.1). Hence, at a confidence interval of 95% (α = 0.05) and power of 98%, the minimum survey sample size required was 120.
Statistical analysis
Statistical analysis was performed using SAS 9.1 or higher version. An independent statistician performed the calculations. The primary dataset was analyzed according to the as-treated population, including all enrolled patients. Significance tests were performed by comparison of the performance goal value (TVF: 9.2%, MACE: 12.5%, TLR: 8.4%, deaths: 1.1% and MI: 3.0%) with the outcome using suitable significance tests: t-test or analysis of variance (ANOVA) and z-test as applicable. Safety analysis included all enrolled population. All drop-outs and patients lost to follow-up were excluded from the safety analysis. Efficacy evaluation was performed for qualifying subjects. All subjects lost to follow-up after 9 months were included for efficacy evaluation based on the last observation carried forward (LOCF) data. For continuous data, the descriptive statistics were used to present data as number of observations, mean, standard deviation (SD), median, minimum and maximum (min.-max.) values, while frequency data were presented as numbers and percentages.
| Results | ▴Top |
Overall, 771 patients were enrolled in the study between April 2014 and March 2021. A detailed patient disposition is given in Figure 1. The mean age of patients was 64.15 ± 10.95 years, and 74.71% were male. Of the total patients, 760, 733, 717, and 691 completed the 1, 9, 12, and 24 months follow-up, respectively. There were two withdrawals, 14 deaths, and 78 patients lost to follow-up within 24 months. The baseline demographics and clinical characteristics of patients are shown in Table 1.
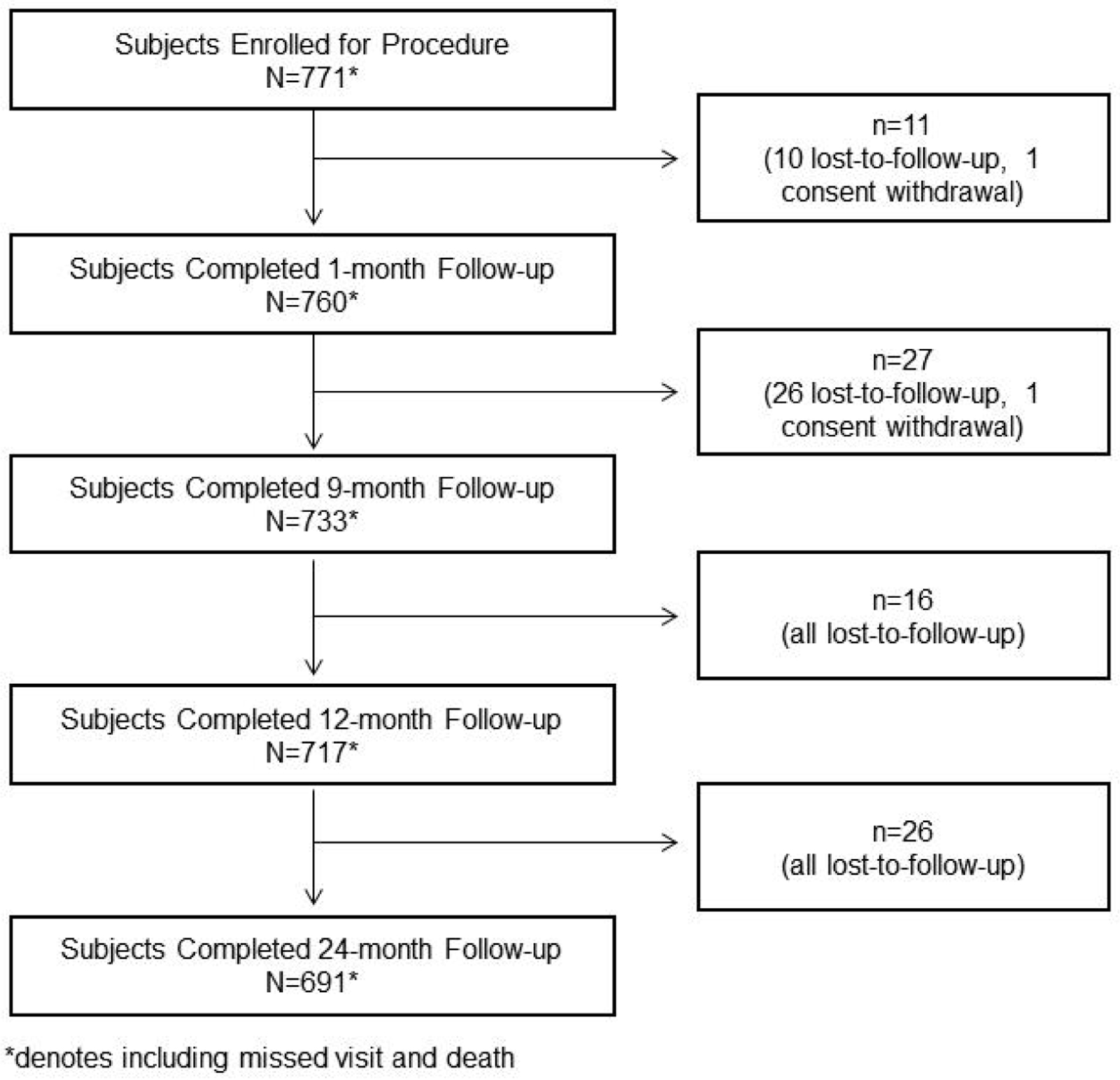 Click for large image | Figure 1. Patient disposition. |
 Click to view | Table 1. Patient Demographics and Baseline Clinical Characteristics |
The total number of lesions treated was 1,079. Most patients had single vessel disease (64.37%), with the left anterior descending (LAD) (42.45%) and right coronary artery (RCA) (28.64%) being the most common vessels involved. Almost all patients had de novo stenosis. The detailed lesion characteristics are shown in Table 2.
 Click to view | Table 2. Lesion Characteristics |
The mean length and diameter of the implanted stents were 25.57 ± 9.35 mm and 3.00 ± 0.44 mm, respectively. The mean minimum lumen diameter before the procedure was 1.0 ± 1.69 mm, which increased to 2.96 ± 1.35 mm after the procedure. The percent of vessel diameter stenosis before the procedure was 83.93 ± 12.84, which decreased to 4.22 ± 11.50 after the procedure. The detailed procedural characteristics are shown in Table 3.
 Click to view | Table 3. Procedural Characteristics |
The cumulative 24-month outcomes are presented in Table 4. The cumulative rates of MACEs were 1.05%, 3.13%, 4.04%, and 5.64% at 1, 9, 12, and 24 months, respectively. The corresponding rates of ST were 0%, 0.13%, 0.28%, and 0.28%, respectively.
 Click to view | Table 4. Cumulative Outcomes After 24 Months Follow-Up |
Outcomes of pre-defined subgroups
Subset 1: subjects who required stents of length > 30 mm
Almost one-third of subjects (32.8%) required stents of length > 30 mm. This subset had a high proportion of patients with diabetes (32%), dyslipidemia (51.38%), and hypertension (67.59%) compared to other subsets (Table 1). Detailed lesion and procedural characteristics are shown in Tables 2 and 3, respectively. The cumulative 24-month outcomes are shown in Table 5. The cumulative rates of MACEs were 0.4%, 4.6%, 5.12%, and 7.01% at 1, 9, 12, and 24 months, respectively. The corresponding rates of ST were 0%, 0.42%, 0.43%, and 0.44%, respectively, indicating the rate of ST was maintained after 9 months.
 Click to view | Table 5. Cumulative Outcomes up to 24 Months Follow-Up for Population Treated With Stent > 30 mm in Length |
Subset 2: subjects who required stents with diameters of 4.00 and 4.50 mm
Among the total cohort, 49 patients required stents of 4 and 4.5 mm in diameter. In this subset, the prevalence of diabetes was quite low at 10.2%; however, the prevalence of peripheral vascular disease was much higher than that in either subset (Table 1). They had a lower prevalence of double vessel disease; however, the RCA was involved in almost two-thirds of patients, which is much higher than that in other subsets (Table 2). The mean lesion length was shorter than that in other subsets at 16.04 ± 6.95 mm. Consequently, the stent length required was also much shorter. The reference vessel diameter (RVD) in this subset was much higher than that in the other subsets at 3.89 ± 0.70 mm (Table 3). The cumulative 24-month outcomes are shown in Table 6. The cumulative rates of MACEs were 0%, 6.25%, 6.25%, and 10.41% at 1, 9, 12, and 24 months, respectively, which was considerably high. However, there was no case of ST until 24 months.
 Click to view | Table 6. Cumulative Clinical Events up to 24 Months for Population Treated With 4.00- and 4.50-mm Diameter Stent |
Subset 3: subjects with bifurcation lesions
There were 82 patients with bifurcation lesions. In this subset, similar to that of the long-stent subset, the prevalence of dyslipidemia was considerably high at 48.78% (Table 1). The major vessel involved was LAD in 56.04% of patients (Table 2). The cumulative 2-year outcomes are shown in Table 7. The cumulative rates of MACEs were 2.46%, 6.32%, 11.53%, and 16.21% at 1, 9, 12, and 24 months, respectively, which was considerably high. The corresponding rates of ST were 0%, 1.27%, 1.28%, and 1.35%, respectively, indicating that the rate of ST was maintained after 9 months.
 Click to view | Table 7. Cumulative Major Cardiac Clinical Events up to 24 Months in Patients With Bifurcation Lesions |
Subset 4: subjects with CTO
Overall, 16.4% of patients had CTO. The basic characteristics are shown in Table 1. This subset had a high thrombus load (severe: 19.15%; moderate: 18.44%) and a high proportion of patients with American Heart Association (AHA) class C lesions (52.48%). Moreover, almost 84% of patients had thrombolysis in myocardial infarction (TIMI) flow 0 (Table 2). The cumulative 2-year outcomes are shown in Table 8. The cumulative rates of MACEs were 0.79%, 5.04%, 6.83%, and 7.07% at 1, 9, 12, and 24 months, respectively. The corresponding rates of ST were 0%, 0.84%, 0.85%, and 0.88%, respectively, indicating that the rate of ST was maintained after 9 months.
 Click to view | Table 8. Cumulative Major Cardiac Events up to 24 Months Follow-Up for Population With CTO |
| Discussion | ▴Top |
The overall rates of ST at 24 months in the current study were very low, both in the overall cohort and in pre-specified patient subsets. Furthermore, ST rates remained consistent across all groups from 9 to 24 months. The cumulative rate of MACEs was 4.04% at 12 months and 5.64% at 24 months. The corresponding rates of ST were 0.28% at both time points. Comparing our results to previous studies, the meriT-2 and meriT-3 trials evaluated the 12-month outcomes of BioMime SES [11, 12]. In the meriT-2 study, which included real-world patients with de novo lesions, the 12-month MACE rate was 6.0% and ST occurred in 0.4% of cases [11]. Our outcomes align with these findings, although meriT-2 excluded patients with complex lesions. In the meriT-3 study, which included all-comer patients who received BioMime SES for the management of CAD, the cumulative 12-month MACE rate was 2.35%, with ST occurring in only 0.09% of cases [12]. These rates were considerably lower than those observed in our study. Specifically, meriT-3 had an average lesion length of 18.5 ± 8.2 mm, whereas our study’s average stent length was 23.1 ± 8.4 mm. Additionally, meriT-3 included 8.9% of patients with CTO, compared to 13.21% in our investigation. In the meriT-V study that compared the XIENCE everolimus-eluting coronary stents with BioMime SES in patients with de novo native coronary artery lesions, the 9-month MACE and ST of the BioMime group were 2.8% and 0%, respectively [13]. These rates were slightly lower than rates observed in our study (3.13% and 0.13%, respectively). However, it is essential to note that meriT-V study had a smaller mean lesion length (16.71 ± 9.74 mm) and mean stent length (21.20 ± 7.73 mm).
Long lesions are a major determinant of unfavorable outcomes [15]. Park et al investigated the efficacy and safety of the Resolute™ zotarolimus-eluting stent in patients with diffuse long coronary lesions (≥ 25 mm) from a prospective, relatively large-scale and real-world registry. The incidence of MACE and definite ST at 1 year was 3.0% and 0.3%, respectively [16]. Other studies involving long stents have shown a 1-year rate of TLF, a composite of cardiac death, target lesion MI, or ischemia-driven TLR ranging from 5% to 14% [17-20]. Park et al also evaluated two different stents (ABT NG DES 48 and XIENCE Skypoint 48 IDE) in subjects with CAD with long de novo native coronary lesions. The rate of 5.8% for cardiac death or all MI at 1 year was observed with ABT NG DES 48 and a TLF rate of 5.7% was observed with XIENCE [21]. These outcomes are consistent with our study findings in the subgroup of patients who required long stents.
The overall 24-month MACE outcomes in our study are comparable or better than those observed over a similar period in other studies (DESSOLVE II [22], COMFORTABLE AMI Trial [23], NEXT [24], BIO-RESORT [25], BIONYX [26]), involving various DESs. However, the rates of ST and cardiac death in our study are the lowest compared to those of other studies [23-26], except the DESSOLVE II study [22]. Notably, the BioMime stent used in our study and the stent used in the DESSOLVE II study had the thinnest struts compared to other studies. These findings underscore the efficacy of thin struts in reducing ST and cardiac mortality.
In a subset of patients requiring stents with diameters of 4 and 4.5 mm, we observed relatively higher rates of MACE outcomes than those of the total cohort and other subgroups, except the subgroup with bifurcation lesions. This was despite a mean lesion length of 16.04 ± 6.95 mm in this subgroup and a large mean RVD of 3.89 ± 0.70 mm. However, there were no cases of ST within this subgroup during the 24-month follow-up. Vessel diameter has been known to affect PCI outcomes, with interventions in small caliber vessels (< 2.5 mm) having limited success [27]. Previous studies have shown that stent diameters > 2.5 mm are associated with more favorable outcomes [27, 28]. However, recent studies on DES with stent diameter as large as 4 and 5 mm are lacking.
In our study, the subgroup of patients with bifurcation lesions exhibited higher MACE rates of 6.32%, 11.53%, and 16.21% at 9, 12, and 24 months, respectively. The corresponding rates of ST were 1.27%, 1.28%, and 1.35%, respectively. Coronary bifurcation PCI is associated with higher rates of restenosis and TLR compared to non-bifurcation lesions. Additionally, the stenting technique employed substantially impacts clinical outcomes [29, 30]. Interestingly, in our study, the 24-month rates of MACE and ST in this subgroup were usually higher than the 24-month rates of MACE and ST reported in recent studies involving patients with bifurcation lesions (POLBOS I+II [31], DIVERGE [32], SPIRIT V [33], DUTCH PEERS [34], TWENTE [35], RESOLUTE [36]). In the SPIRIT V, which had much lower rates of MACE at 24 months (11.3%), the mean lesion length was 16.0 ± 6.5 mm, while it was 21.32 ± 10.22 mm in our study. Furthermore, in SPIRIT V study, 80.3% of patients had a baseline TIMI flow grade of III, compared to 64.84% in our study [33]. Notably, our study allowed single or two-stent strategies at the operator’s discretion, which may have contributed to the poor outcomes observed in the bifurcation lesions group compared to other studies.
PCI for CTO is associated with a lower success rate, uncertain benefits, and a higher rate of complications. Moreover, the differences in techniques between CTO and non-CTO PCI have consistently led to debates about the long-term outcomes and vessel patency even after successful CTO PCI [37]. In our study, the subgroup of patients with CTO demonstrated the cumulative MACE rates of 6.83% and 7.07% at 1 and 2 years, respectively, with the corresponding ST rates of 0.85% and 0.88%, respectively. Remarkably, these outcomes were observed despite a high thrombus load and a high proportion of patients with AHA class C lesions in this subgroup. Moreover, nearly 84% of patients had a baseline TIMI flow 0. Comparing our results to recent studies, a recent retrospective analysis of patients who underwent LAD CTO PCI at a high-volume single center reported a 2-year MACE rate of 15% [38]. Another study examining the long-term outcomes reported a 3.6-year MACE rate of 19.1% [39]. Therefore, our outcomes in this subgroup appear more favorable than those reported in these recent studies.
Table 9 shows the comparison of clinical outcomes of BioMime SES system to other stents, such as the Xience, Resolute, MiStent, Endeavor Sprint, Biolimus, and the Gazelle bare metal stent. Notably, the BioMime stent demonstrates a favorable safety profile with relatively low incidences of cardiac death (0.87%), TVR (3.32%), and ST (0.28%) compared to other stents like the Resolute and Gazelle bare metal stent, which reported higher rates of adverse events. This comparison underscores the competitive efficacy of the BioMime stent in reducing MACE and improving patient outcomes [26, 40-43].
 Click to view | Table 9. Clinical Outcomes Comparison of BioMime Stent With Other Drug-Coated/Bare Metal Stents |
Limitations
This study has several limitations. Firstly, it was a non-randomized single-arm study without an active control group. Secondly, procedural and technical dissimilarities in complex lesions (bifurcation and CTO) may have contributed to non-uniform outcomes within these subgroups. Additionally, the 24-month follow-up period might not provide sufficient time to thoroughly assess the safety and performance of the BioMime SES. Consequently, longer follow-up periods are warranted. Lastly, it is important to note that outcomes were not confirmed by angiography or by any other investigation methods.
Conclusion
The BioMime SES with ultra-thin struts and a biodegradable polymer demonstrated favorable safety and efficacy outcomes over a 2-year period in a real-world all-comers CAD patient population. Notably, the overall rates of MACE and ST were low. However, subgroup analyses revealed higher MACE rates in patients with bifurcation lesions and those receiving larger stent diameters, despite the relatively low ST rate.
| Supplementary Material | ▴Top |
Suppl 1. Eligibility criteria.
Acknowledgments
None to declare.
Financial Disclosure
MILES Global Registry was sponsored by Meril Life Sciences Pvt. Ltd, India.
Conflict of Interest
Martin Hudec reports grant/research support from Meril Life Sciences. Alexander J.J. Ijsselmuiden reports consulting fees from Meril Life Sciences, Medtronic, Angiocare, Keystone Heart, PulseCath, Salveo, Abbott, Philips, Fysicon, Cardiawave, Pi Medical, and Svelte. Ramiro Trillo serves as a proctor for Medtronic and Boston Scientific. Hyeon-Cheol Gwon received an institutional research grant from Abbott Vascular, Boston Scientific, Genoss and Medtronic, Inc. Yi-Chih Wang received study funding from Daiichi Sankyo. Min-Ji Charng reports honoraria from AstraZeneca, Merck Sharp & Dohme, Pfizer, Amgen, and Sanofi. Bela Merkely reports grants from Boston Scientific, NRDIF Hungary, National Heart Program; personal fees from Biotronik, Abbott, Astra Zeneca, Novartis, and Boehringer-Ingelheim; and grants from Medtronic outside the submitted work. Beatriz Vaqueizo Montilla: support from Boston Scientific, Medtronic, Lifetech. Other authors have no conflict of interest to disclose.
Informed Consent
Informed consent was obtained from all the subjects included in this registry.
Author Contributions
Conceptualization: MH, AJJI, and BM; methodology: MH, AJJI, and BM; formal analysis: MH, AJJI, and BM; investigation: all authors; resources: all authors; data curation: all authors; writing - original draft: all authors; writing - review and editing: all authors; visualization: all authors; supervision: MH; project administration: MH and AJJI; funding acquisition: all authors.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
AHA: American Heart Association; ARC: Academic Research Consortium; CABG: coronary artery bypass graft; CAD: coronary artery disease; CoCr: cobalt chromium; CTO: chronic total occlusion; DES: drug-eluting stent; ECG: electrocardiogram; ISR: in-stent restenosis; LAD: left anterior descending; LOCF: last observation carried forward; MACE: major adverse cardiovascular event; MI: myocardial infarction; PCI: percutaneous coronary intervention; PLGA: poly lactic-co-glycolic acid; PLLA: poly-L-lactic acid; PtCr: platinum chromium; RCA: right coronary artery; RVD: reference vessel diameter; SD: standard deviation; SES: sirolimus-eluting stent; ST: stent thrombosis; TIMI: thrombolysis in myocardial infarction; TLR: target lesion revascularization; TVF: target vessel failure; TVR: target vessel revascularization
| References | ▴Top |
- Condello F, Spaccarotella C, Sorrentino S, Indolfi C, Stefanini GG, Polimeni A. Stent thrombosis and restenosis with contemporary drug-eluting stents: predictors and current evidence. J Clin Med. 2023;12(3):1238.
doi pubmed - Polimeni A, Sorrentino S, Spaccarotella C, Mongiardo A, Sabatino J, De Rosa S, Gori T, et al. Stent thrombosis after percutaneous coronary intervention: from bare-metal to the last generation of drug-eluting stents. Interv Cardiol Clin. 2022;11(4):465-473.
doi pubmed - Alfonso F, Coughlan JJ, Giacoppo D, Kastrati A, Byrne RA. Management of in-stent restenosis. EuroIntervention. 2022;18(2):e103-e123.
doi pubmed - Madhavan MV, Howard JP, Naqvi A, Ben-Yehuda O, Redfors B, Prasad M, Shahim B, et al. Long-term follow-up after ultrathin vs. conventional 2nd-generation drug-eluting stents: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J. 2021;42(27):2643-2654.
doi pubmed - Iannaccone M, Gatti P, Barbero U, Bassignana A, Gallo D, de Benedictis M, Helft G, et al. Impact of strut thickness and number of crown and connectors on clinical outcomes on patients treated with second-generation drug eluting stent. Catheter Cardiovasc Interv. 2020;96(7):1417-1422.
doi pubmed - Morice MC, Colombo A, Meier B, Serruys P, Tamburino C, Guagliumi G, Sousa E, et al. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions: the REALITY trial: a randomized controlled trial. JAMA. 2006;295(8):895-904.
doi pubmed - Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193-202.
doi pubmed - Serruys PW, Onuma Y, Garg S, Vranckx P, De Bruyne B, Morice MC, Colombo A, et al. 5-year clinical outcomes of the ARTS II (Arterial Revascularization Therapies Study II) of the sirolimus-eluting stent in the treatment of patients with multivessel de novo coronary artery lesions. J Am Coll Cardiol. 2010;55(11):1093-1101.
doi pubmed - Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, Jeger R, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48(12):2584-2591.
doi pubmed - Dani S, Costa RA, Joshi H, Shah J, Pandya R, Virmani R, Sheiban I, et al. First-in-human evaluation of the novel BioMime sirolimus-eluting coronary stent with bioabsorbable polymer for the treatment of single de novo lesions located in native coronary vessels - results from the meriT-1 trial. EuroIntervention. 2013;9(4):493-500.
doi pubmed - Seth A, Wander GS, Mullasari A, Nanjappa MC, Heggunje-Shetty P, Alexander T, et al. Late angiographic and clinical outcomes of the novel BioMime™ sirolimus-eluting coronary stent with ultra-thin cobalt-chromium platform and biodegradable polymer for the treatment of diseased coronary vessels: results from the prospective, multicentre meriT-2 clinical trial. Asia Intervention. 2016;2:19-27.
- Jain RK, Chakravarthi P, Shetty R, Ramchandra P, Polavarapu RS, Wander GS, Mohan B, et al. One-year outcomes of a BioMime Sirolimus-Eluting Coronary Stent System with a biodegradable polymer in all-comers coronary artery disease patients: The meriT-3 study. Indian Heart J. 2016;68(5):599-603.
doi pubmed - Abizaid A, Kedev S, Kedhi E, Talwar S, Erglis A, Hlinomaz O, Masotti M, et al. Randomised comparison of a biodegradable polymer ultra-thin sirolimus-eluting stent versus a durable polymer everolimus-eluting stent in patients with de novo native coronary artery lesions: the meriT-V trial. EuroIntervention. 2018;14(11):e1207-e1214.
doi pubmed - Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58(24):e44-122.
doi pubmed - Kang DY, Jang JS, Chang M, Lee CH, Lee PH, Ahn JM, Lee SW, et al. Comparison of different types of drug-eluting stents for de novo long coronary artery lesions. JACC Asia. 2022;2(4):446-456.
doi pubmed - Park KH, Ahn Y, Koh YY, Ki YJ, Kim SS, Kim HK, Choi DH, et al. Effectiveness and safety of Zotarolimus-Eluting Stent (Resolute Integrity) in patients with diffuse long coronary artery disease. Korean Circ J. 2019;49(8):709-720.
doi pubmed - Patra S, Chakraborty RN, Pande A, Banerjee S, Jena M, Mandal PC, De SK, et al. Zotarolimus-eluting Resolute Integrity versus everolimus-eluting Xience Xpedition stents in the management of very long (>30mm) de novo coronary artery stenosis. Cardiovasc Revasc Med. 2017;18(3):160-164.
doi pubmed - Lee M, Hiremath S, Zambahari R, Leon M, Mauri L, Yeung A, Resolute US, et al. One-year outcomes of percutaneous coronary intervention with the 38-mm Resolute zotarolimus-eluting stent. Am J Cardiol. 2013;112(9):1335-1341.
doi pubmed - Bahuleyan CG, Krishna Kumar VV, Babu S. Prospective study to evaluate safety and efficacy of Zotarolimus Eluting Stent (PSEZES) in patients with long coronary artery lesions. Indian Heart J. 2015;67(3):233-238.
doi pubmed - Ahn JM, Park DW, Kim YH, Song H, Cho YR, Kim WJ, Lee JY, et al. Comparison of resolute zotarolimus-eluting stents and sirolimus-eluting stents in patients with de novo long coronary artery lesions: a randomized LONG-DES IV trial. Circ Cardiovasc Interv. 2012;5(5):633-640.
doi pubmed - Park KE, Pingle SC, Chang CJ. LB-3 Clinical outcomes of the XIENCE Skypoint 48mm drug-eluting stent in long coronary artery lesions: primary endpoint data from the SPIRIT 48 trial. JSCAI. 2023;2(3).
- Wijns W, Vrolix M, Verheye S, Schoors D, Slagboom T, Gosselink M, Benit E, et al. Randomised study of a bioabsorbable polymer-coated sirolimus-eluting stent: results of the DESSOLVE II trial. EuroIntervention. 2015;10(12):1383-1390.
doi pubmed - Raber L, Kelbaek H, Taniwaki M, Ostojic M, Heg D, Baumbach A, von Birgelen C, et al. Biolimus-eluting stents with biodegradable polymer versus bare-metal stents in acute myocardial infarction: two-year clinical results of the COMFORTABLE AMI trial. Circ Cardiovasc Interv. 2014;7(3):355-364.
doi pubmed - Natsuaki M, Kozuma K, Morimoto T, Shiomi H, Kimura T. Two-year outcome of a randomized trial comparing second-generation drug-eluting stents using biodegradable or durable polymer. JAMA. 2014;311(20):2125-2127.
doi pubmed - Kok MM, Zocca P, Buiten RA, Danse PW, Schotborgh CE, Scholte M, Hartmann M, et al. Two-year clinical outcome of all-comers treated with three highly dissimilar contemporary coronary drug-eluting stents in the randomised BIO-RESORT trial. EuroIntervention. 2018;14(8):915-923.
doi pubmed - Buiten RA, Ploumen EH, Zocca P, Doggen CJM, Jessurun GAJ, Schotborgh CE, Roguin A, et al. Thin Composite-Wire-Strut Zotarolimus-eluting stents versus ultrathin-strut sirolimus-eluting stents in BIONYX at 2 years. JACC Cardiovasc Interv. 2020;13(9):1100-1109.
doi pubmed - Plitt A, Claessen BE, Sartori S, Baber U, Chandrasekhar J, Aquino M, Vijay P, et al. Impact of stent diameter on outcomes following percutaneous coronary intervention with second-generation drug-eluting stents: Results from a large single-center registry. Catheter Cardiovasc Interv. 2020;96(3):558-564.
doi pubmed - Hara H, Ono M, Kawashima H, Kogame N, Mack MJ, Holmes DR, Morice MC, et al. Impact of stent length and diameter on 10-year mortality in the SYNTAXES trial. Catheter Cardiovasc Interv. 2021;98(3):E379-E387.
doi pubmed - Albiero R, Burzotta F, Lassen JF, Lefevre T, Banning AP, Chatzizisis YS, Johnson TW, et al. Treatment of coronary bifurcation lesions, part I: implanting the first stent in the provisional pathway. The 16th expert consensus document of the European Bifurcation Club. EuroIntervention. 2022;18(5):e362-e376.
doi pubmed - Lassen JF, Albiero R, Johnson TW, Burzotta F, Lefevre T, Iles TL, Pan M, et al. Treatment of coronary bifurcation lesions, part II: implanting two stents. The 16th expert consensus document of the European Bifurcation Club. EuroIntervention. 2022;18(6):457-470.
doi pubmed - Gil RJ, Kern A, Formuszewicz R, Inigo Garcia LA, Dobrzycki S, Vassilev D, Bil J. 6-year results of BiOSS stents in coronary bifurcation treatment. Eur J Clin Invest. 2021;51(8):e13555.
doi pubmed - Buysschaert I, Dubois CL, Dens J, Ormiston J, Worthley S, McClean D, Ottervanger JP, et al. Three-year clinical results of the Axxess Biolimus A9 eluting bifurcation stent system: the DIVERGE study. EuroIntervention. 2013;9(5):573-581.
doi pubmed - Dzavik V, Kaul U, Guagliumi G, Chevalier B, Smits PC, Stuteville M, Li D, et al. Two-year outcomes after deployment of XIENCE V everolimus-eluting stents in patients undergoing percutaneous coronary intervention of bifurcation lesions: a report from the SPIRIT V single arm study. Catheter Cardiovasc Interv. 2013;82(3):E163-172.
doi pubmed - van der Heijden LC, Kok MM, Lam MK, Danse PW, Schramm AR, Jessurun GA, Tjon Joe Gin RM, et al. Bifurcation treatment with novel, highly flexible drug-eluting coronary stents in all-comers: 2-year outcome in patients of the DUTCH PEERS trial. Clin Res Cardiol. 2016;105(3):206-215.
doi pubmed - Lam MK, Sen H, van Houwelingen KG, Lowik MM, van der Heijden LC, Kok MM, de Man FH, et al. Three-year clinical outcome of patients with bifurcation treatment with second-generation Resolute and Xience V stents in the randomized TWENTE trial. Am Heart J. 2015;169(1):69-77.
doi pubmed - Diletti R, Garcia-Garcia HM, Bourantas CV, van Geuns RJ, Van Mieghem NM, Vranckx P, Zhang YJ, et al. Clinical outcomes after zotarolimus and everolimus drug eluting stent implantation in coronary artery bifurcation lesions: insights from the RESOLUTE All Comers Trial. Heart. 2013;99(17):1267-1274.
doi pubmed - Almarzooq ZI, Tamez H, Wang Y, Curtis JP, Kirtane AJ, Secemsky EA, Valsdottir LR, et al. Long-term outcomes of chronic total occlusion percutaneous coronary intervention among medicare beneficiaries. J Soc Cardiovasc Angiogr Interv. 2023;2(2):100584.
doi pubmed - Megaly M, Zakhour S, Karacsonyi J, Basir MB, Kunkel K, Gupta A, Neupane S, et al. Outcomes of chronic total occlusion percutaneous coronary intervention of the left anterior descending artery. Am J Cardiol. 2023;193:75-82.
doi pubmed - Guo L, Zhang X, Lv H, Zhong L, Wu J, Ding H, Xu J, et al. Long-term outcomes of successful revascularization for patients with coronary chronic total occlusions: a report of 1,655 patients. Front Cardiovasc Med. 2020;7:116.
doi pubmed - Kiatchoosakun S, Pienvichit P, Kuanprasert S, Suraphakdee N, Phromminikul A. A clinical evaluation of the XIENCE V everolimus eluting stent in the treatment of patients with coronary artery disease: Result from Thailand Registry - XIENCE V performance evaluation (THRIVE study). Indian Heart J. 2017;69(2):165-169.
doi pubmed - Meredith IT, Worthley SG, Whitbourn R, Walters D, McClean D, Ormiston J, Horrigan M, et al. Long-term clinical outcomes with the next-generation Resolute Stent System: a report of the two-year follow-up from the RESOLUTE clinical trial. EuroIntervention. 2010;5(6):692-697.
doi pubmed - Wijns W, Suttorp MJ, Zagozdzon L, Morice MC, McClean D, Stella P, Donohoe D, et al. Evaluation of a crystalline sirolimus-eluting coronary stent with a bioabsorbable polymer designed for rapid dissolution: two-year outcomes from the DESSOLVE I and II trials. EuroIntervention. 2016;12(3):352-355.
doi pubmed - Raber L, Kelbaek H, Ostojic M, Baumbach A, Heg D, Tuller D, von Birgelen C, et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308(8):777-787.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.