| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 2, April 2025, pages 130-139
Safety and Performance of the MOZEC Sirolimus-Eluting Coronary Balloon in the Treatment of Stenotic Coronary Artery Lesions: A Real-World, Multicenter, Post-Marketing Surveillance Study
Rajendra Kumar Premchand Jaina, j , Keyur Parikhb, Selvamani Sethuramanc, Kamal Sharmad, Sanjeeb Roye, Suryaprakasa Rao Vithalaf, Kondal Rao Gollamandalaf, Gobu Packirisamyg, Sai Sudhakar Mantravadih, Tomasz Rolederi
aDepartment of Cardiology, Krishna Institute of Medical Sciences, Secunderabad, Telangana, India
bDepartment of Cardiology, Care Institute of Medical Sciences, Ahmedabad, Gujarat, India
cDepartment of Cardiology, Meenakshi Mission Hospital and Research Centre, Madurai, Tamil Nadu, India
dDepartment of Cardiology, Sanjivani Superspeciality Hospitals Pvt. Ltd., Ahmedabad, Gujarat, India
eDepartment of Cardiology, Fortis Escorts Hospital, Jaipur, Rajasthan, India
fDepartment of Cardiology, Apollo Hospitals, Hyderabad, Telangana, India
gDepartment of Cardiology, Global Health City, Chennai, Tamil Nadu, India
hDepartment of Cardiology, Gleneagles Global Hospitals, Hyderabad, Telangana, India
iDepartment of Non-Surgical Clinical Sciences, Faculty of Medicine, Wroclaw University of Science and Technology (WUST), Wroclaw, Poland
jCorresponding Author: Rajendra Kumar Premchand Jain, Department of Cardiology, Krishna Institute of Medical Sciences, Secunderabad 500003, Telangana, India
Manuscript submitted December 5, 2024, accepted February 8, 2025, published online February 28, 2025
Short title: MOZEC SEB: Real-World Post-Marketing Study
doi: https://doi.org/10.14740/cr2026
| Abstract | ▴Top |
Background: Drug-eluting balloons, surface-coated with antiproliferative agents such as sirolimus or paclitaxel, have emerged as an alternative therapeutic option for coronary stenosis. This study evaluated safety and effectiveness of the MOZEC sirolimus-eluting percutaneous transluminal coronary angioplasty (PTCA) balloon dilation catheter (Meril Life Sciences Pvt. Ltd., India) across diverse clinical scenarios in coronary artery stenosis treatment.
Methods: A prospective, single-arm, multicenter, real-world, post-marketing surveillance study evaluated the safety and performance of the MOZEC sirolimus-eluting balloon (SEB) in treating native coronary artery disease in daily clinical practice. Patients were followed for 24 months, with clinical visits or telephonic calls at 1, 6, 12, and 24 months after the index procedure. Safety endpoints included major adverse cardiac events (MACEs), and performance endpoints include change in late lumen loss, clinical success, and device success.
Results: A total of 141 patients were enrolled in the study. The MOZEC SEB was used in 127 (70.17%) de novo lesions, 40 (22.1%) in-stent restenosis lesions, and 14 (7.73%) bifurcations lesions. Over the 24-month follow-up period (n = 134), six cumulative MACEs (4.47%) were observed, comprising two cardiac deaths (1.49%), five myocardial infarctions (3.73%), and four target lesion revascularizations (2.99%). Late lumen loss analysis included 17 patients who underwent additional coronarography at the 6-month follow-up. In-segment and in-device late lumen loss at 6-month follow-up was 0.14 ± 0.37 mm.
Conclusions: The application of MOZEC SEB in various clinical scenarios demonstrated safety and efficacy over long-term follow-up. These findings align with the favorable vessel healing observed during the 6-month imaging follow-up.
Keywords: Coronary artery disease; Drug-eluting balloon; In-stent restenosis; Sirolimus-eluting balloon
| Introduction | ▴Top |
Coronary artery disease places a significant economic burden in the whole world as it results in over 7 million deaths and 129 million disability-adjusted life years per year [1]. Percutaneous coronary interventions (PCIs) have become a cornerstone in the global treatment of coronary artery diseases. The gold standard involves the dilatation of coronary artery stenosis followed by the deployment of a metal stent to maintain vessel patency. While advancements in antimitotic drug-eluting stents (DESs) have significantly reduced the incidence of restenosis rates over the past decade; however long-term follow-up still presents questionable results because adverse cardiovascular events related to device failure were found similar in DES, as well as bare metal stents [2]. Despite these advancements, stent implantation encounters limitations, particularly in addressing in-stent restenosis (ISR), treating small vessels, and managing bifurcation lesions [3-5]. In light of these challenges, drug-eluting balloons (DEBs), surface-coated with antiproliferative agents such as sirolimus or paclitaxel, have emerged as an alternative therapeutic option for coronary stenosis [6, 7]. Initially conceptualized for ISR management, DEBs facilitate homogeneous drug delivery across the vessel wall, effectively mitigating neointimal proliferation [8, 9]. The intravascular imaging has also showed favorable vessel healing after DEB application [10].
The introduction of DEB offers several advantages over DES, including reduced late-stage inflammatory responses, restenosis, and thrombosis rates [7]. Notably, DEBs lack a permanent metallic scaffold, and durable polymer matrix, preserving vascular anatomy, minimizing hemodynamic disruptions, and shortening the required duration of dual antiplatelet therapy (DAPT) [11-14]. These attributes make DEBs particularly advantageous for the treatment of native coronary artery stenosis [15]. Recent developments have introduced new sirolimus-eluting balloons (SEBs) specifically designed for the treatment of patients with native coronary artery, encompassing total occlusions and primary lesions in acute myocardial infarction (MI), as well as for post-dilatation of balloon-expandable stents [7, 16]. In this context, the present study aims to evaluate patient outcomes, assessing the safety and efficacy of the MOZEC sirolimus-eluting percutaneous transluminal coronary angioplasty (PTCA) balloon dilatation catheter (Meril Life Sciences Pvt. Ltd., India) across varied clinical scenarios in the treatment of coronary artery stenosis.
| Materials and Methods | ▴Top |
Study design
This prospective, single-arm, multicenter, real-world, post-marketing surveillance study evaluated the safety and effectiveness of the MOZEC sirolimus-eluting PTCA balloon dilatation catheter for the treatment of native coronary artery disease in daily clinical practice between June 2017, and August 2021. The study was registered with the Clinical Trials Registry of India (CTRI/2017/03/008002).
Study device
The CE-marked MOZEC SEB is based on drug delivery system featuring a unique formulation of solid lipid substrates (SLS) containing sirolimus (3.0 µg/mm2). It has a stable drug formulation with controlled, targeted drug release and prolonged tissue residence time. The SLS have biodegradable lipid particles and excellent biocompatibility. The deliverability of the PTCA catheter (length: 142 cm) relies on a polytetrafluoroethylene-coated proximal shaft (diameter: 1.98 F) with a low-tip profile and a semi-compliant balloon to cross challenging lesions. The sizes (diameter) available are 2.00, 2.25, 2.50, 2.75, 3.00, 3.50, 4.00, and 4.50 mm. The nominal pressure required to deflate the balloon is 7 atm for all diameters with rated burst pressure (RBP) 16 atm for diameter size 2.00 to 4.00 mm 14 atm for diameter size 4.50 mm. The features of the device are shown in Figure 1.
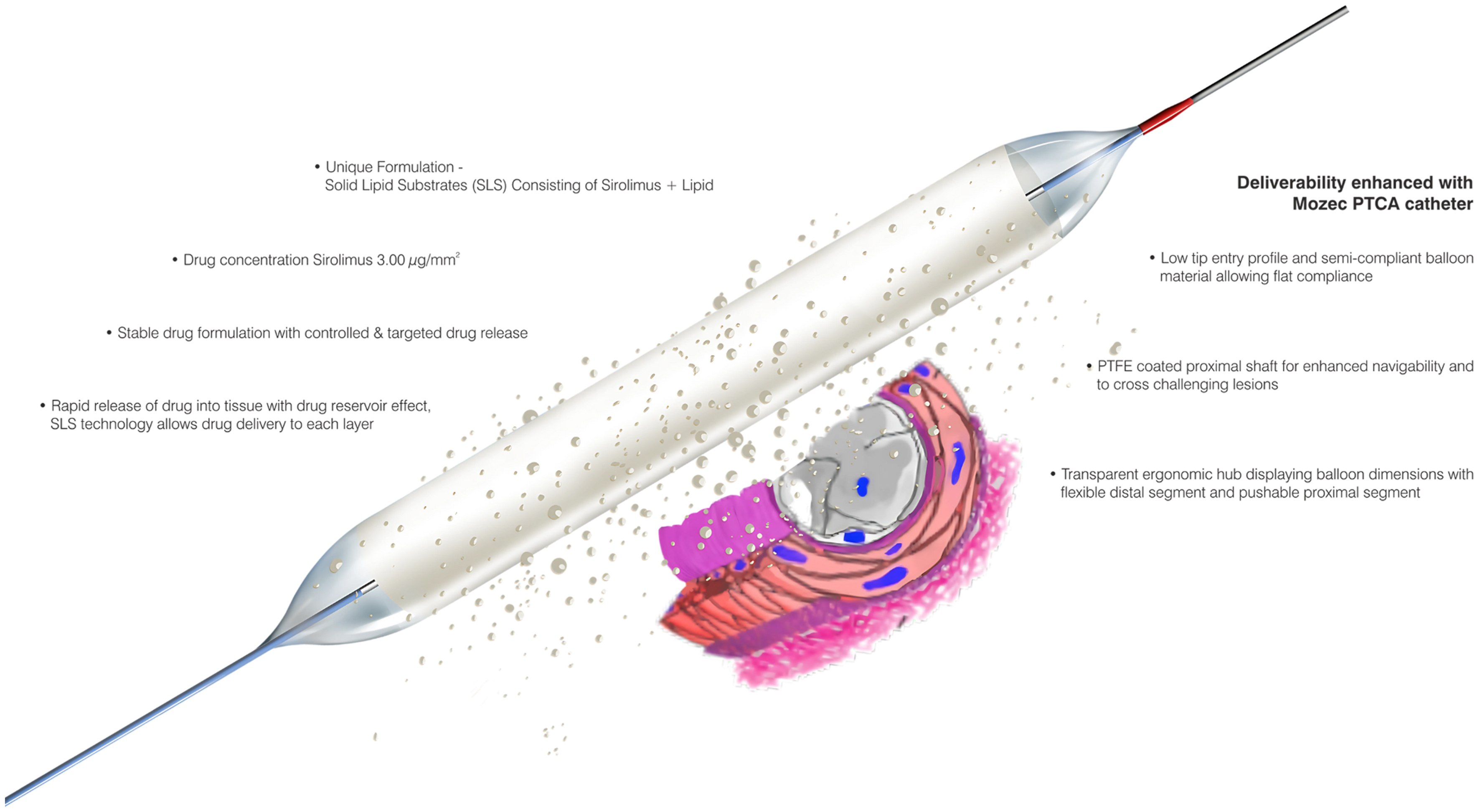 Click for large image | Figure 1. The MOZEC SEB showing its salient features and drug release mechanism. PTFE: polytetrafluoroethylene SEB: sirolimus-eluting balloon. |
Patient population
The inclusion criteria included patients aged ≥ 18 years with target lesions in native coronary arteries, ranging in diameter from 2.00 mm to 4.50 mm, responsible for acute MI, total coronary occlusions, and ISR. The enrolled lesions exhibited stenosis levels between ≥ 50% and ≤ 100% and lengths of ≤ 41 mm, as visually estimated. The treated lesion had to be covered by a single MOZEC SEB dilatation. Exclusion criteria included cardiogenic shock, unprotected left main disease, lesions requiring rotational atherectomy, coronary graft lesions, prior brachytherapy, life expectancy < 2 years, serum creatinine level > 2.0 mg/dL or 160 µmol/L, platelet count < 100,000 cells/mm3 or > 700,000 cells/mm3, patients on cytostatic or radiation therapy, allergy to anticoagulation/antiplatelet therapy, history of stroke within the prior 6 months, medical history of allergy to aspirin or sirolimus, active peptic ulcer or upper gastrointestinal bleeding within the past 6 months, and breastfeeding women.
Ethical statement
All patients signed the informed consent form prior to their enrollment in the trial. The independent ethics committees of each participating institution approved the trial protocol and supervised the ethical conduct of the trial at each clinical site. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Procedural details
The PCI technique was performed in accordance with the standard American College of Cardiology (ACC)/American Heart Association (AHA) guidelines. Vascular access via radial or femoral artery was chosen, as per physician’s discretion. The procedure involved inserting a guidewire through the hemostatic valve connector under fluoroscopy, followed by crossing the lesion using the accepted PCI techniques. Pre-dilatation was performed using a balloon catheter, with the radiopaque markers aiding in accurate positioning and placement. Stenotic lesions were dilated, and the MOZEC SEB was inflated at a nominal pressure of 7 atm for a minimum of 30 s to facilitate drug delivery. In cases of multiple inflations, the duration was extended up to 60 s. The standard antiplatelet/anticoagulation regimen was provided based on the ACC/AHA guidelines or at the investigator’s discretion. Bailout stenting was advised as per the discretion of the treating interventional cardiologist in case of requirement.
Study flow
All patients were followed for 24 months through clinical visits or telephonic calls at 1, 6, 12 and 24 months after the index procedure. Details on follow-up schedule and assessments are summarized here (Supplementary Material 1, cr.elmerpub.com). As per the protocol, 30% (n = 37) of the target population was planned for late lumen loss (LLL) analysis; however, due to logistic/administrative constraints, a total of 17 patients underwent LLL analysis at 6-month follow-up.
Study endpoints
The primary safety endpoint was the analysis of major adverse cardiac events (MACEs) within a 24-month follow-up period after the application of MOZEC SEB. MACE was defined as a composite outcome comprising cardiac death, MI, and target lesion revascularization (TLR). TLR was described as any repeated PCI or bypass surgery of the target lesion due to complications.
Secondary study endpoints included the assessment of LLL, which was defined as the difference between the post-procedural minimum luminal diameter and the follow-up minimum luminal diameter at 6 months, as determined by quantitative coronary angiography (QCA). Clinical success was defined as procedural success without any complications (such as death, thrombosis of the TLR, or target vessel revascularization (TVR)) prior to discharge.
Device success was determined by successful delivery, balloon inflation, and deflation without bursting below the RBP. Additionally, a user rating on technical properties was recorded. User satisfaction was measured on a scale from 0 to 5, considering flexibility, trackability, pushability, crossability, inflation time, deflation time, radiopaque marker visibility, and ease of balloon removal. The scores were defined as follows: 0 = very poor, 1 = poor, 2 = below average, 3 = average, 4 = good, and 5 = excellent.
Statistical analysis
Data distribution was evaluated using the Kolmogorov-Smirnov test. Normally distributed data were expressed as mean ± standard deviation (SD), while non-normally distributed data were presented as median with interquartile ranges (IQR, 25th to 75th percentile). A paired t-test was used for analyzing the normally distributed data, and the Mann-Whitney U test was employed for non-normally distributed data. Categorical data were assessed using Fisher’s exact test or the Chi-square test. A two-tailed P value of less than 0.05 was considered statistically significant. Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 22.0 (IBM, Armonk, New York, USA).
| Results | ▴Top |
Patient characteristics
A total of 141 patients were enrolled in the study. Figure 2 depicts the patient’s disposition. The study cohort was predominantly male, comprising 77.30% of the participants. Most suffered from hypertension (66.67%) and diabetes mellitus (60.28%). The distribution of coronary artery disease was as follows: single-vessel disease in 39.72% (n = 56), double-vessel disease in 29.08% (n = 41), and multi-vessel disease (≥ triple vessel disease) in 31.20% (n = 44) of the patients. The baseline demographic characteristics and medical history of the study patients are presented in Table 1.
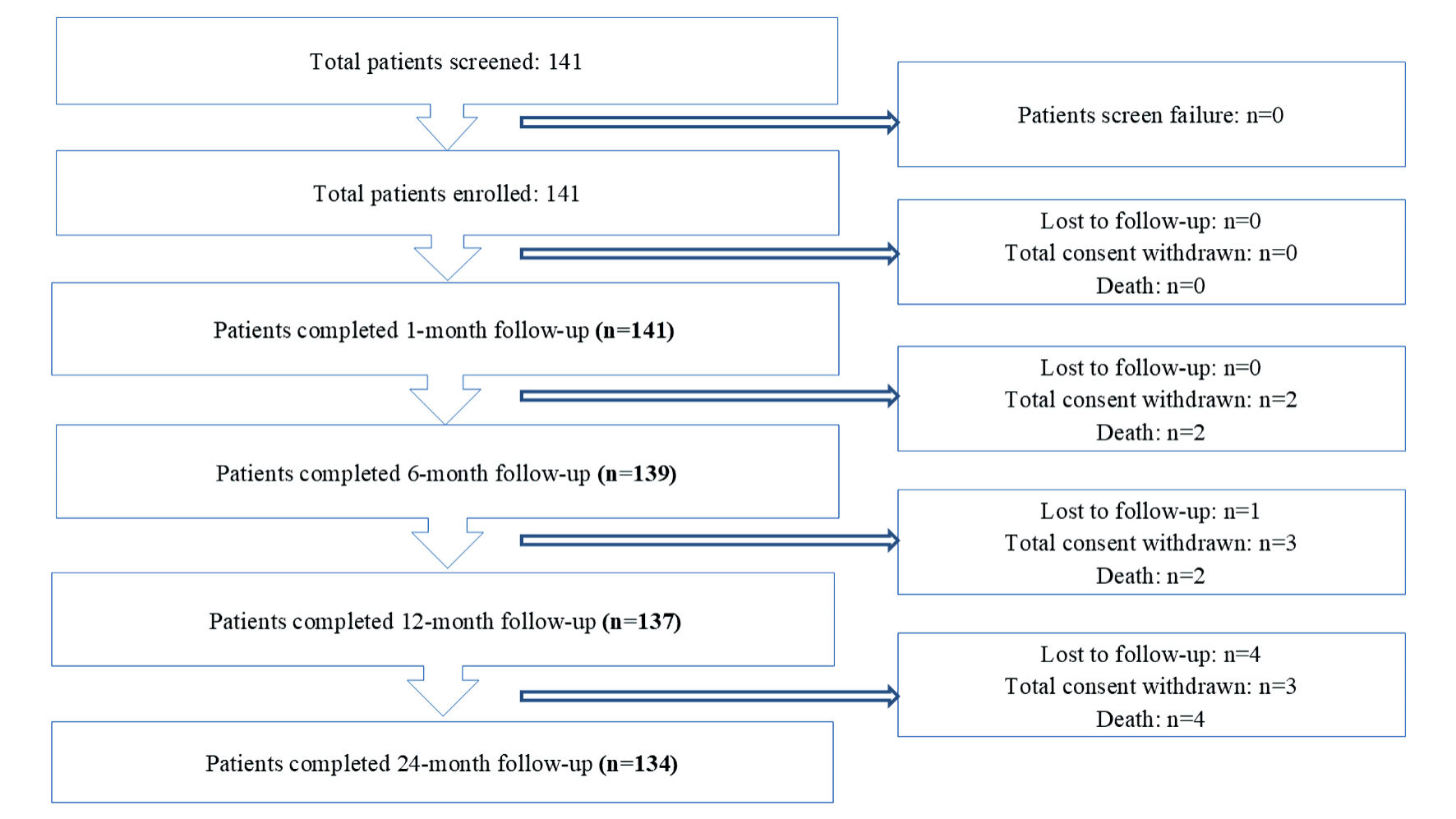 Click for large image | Figure 2. Disposition table. |
 Click to view | Table 1. Baseline Patient Characteristics |
Lesion characteristics
The MOZEC SEB balloon was used in 127 (70.17%) de novo lesions, 40 (22.10%) cases of ISR, and 14 (7.73%) bifurcations. Lesions were classified as type A1 in 42 cases (23.20%), type B1 in 31 cases (17.13%), type B2 in 35 cases (19.34%), and type C in 73 cases (40.33%). Mild calcifications were detected in 108 lesions (59.67%), moderate in nine lesions (4.97%), and severe in three lesions (1.66%). The location of the lesions is presented in Table 2.
 Click to view | Table 2. Location and Clinical Features of the Lesions |
Procedure details
Radial access was the most common vascular access, which was used in 111 cases (78.72%). The average diameter of the applied MOZEC SEB balloon was 2.54 ± 0.41 mm, with an average length of 20.68 ± 9.16 mm. Post-procedure Thrombolysis in Myocardial Infarction (TIMI) flow grades were TIMI 2 in one case (0.55%) and TIMI 3 in 180 cases (99.45%). Clinical features of the lesions are summarized in Table 2. Device success and clinical success were observed in 100% of cases. There were no instances of bailout stenting.
Clinical outcomes
During the 24-month follow-up, a total of six cumulative MACEs were observed (4.47%). Among these, four patients died (2.99%) with two of those deaths attributed to cardiac deaths (1.49%). Additionally, five patients experienced MI (3.73%), and four underwent TLR (2.99%) (Table 3). The Kaplan-Meier curve illustrating the overall survival rate at 24-month follow-up is presented in Figure 3. According to Figure 3, the survival probability was more in de novo group as compared to the ISR group at the end of 24 months (97.65% vs 93.10%) follow-up.
 Click to view | Table 3. Safety Outcomes |
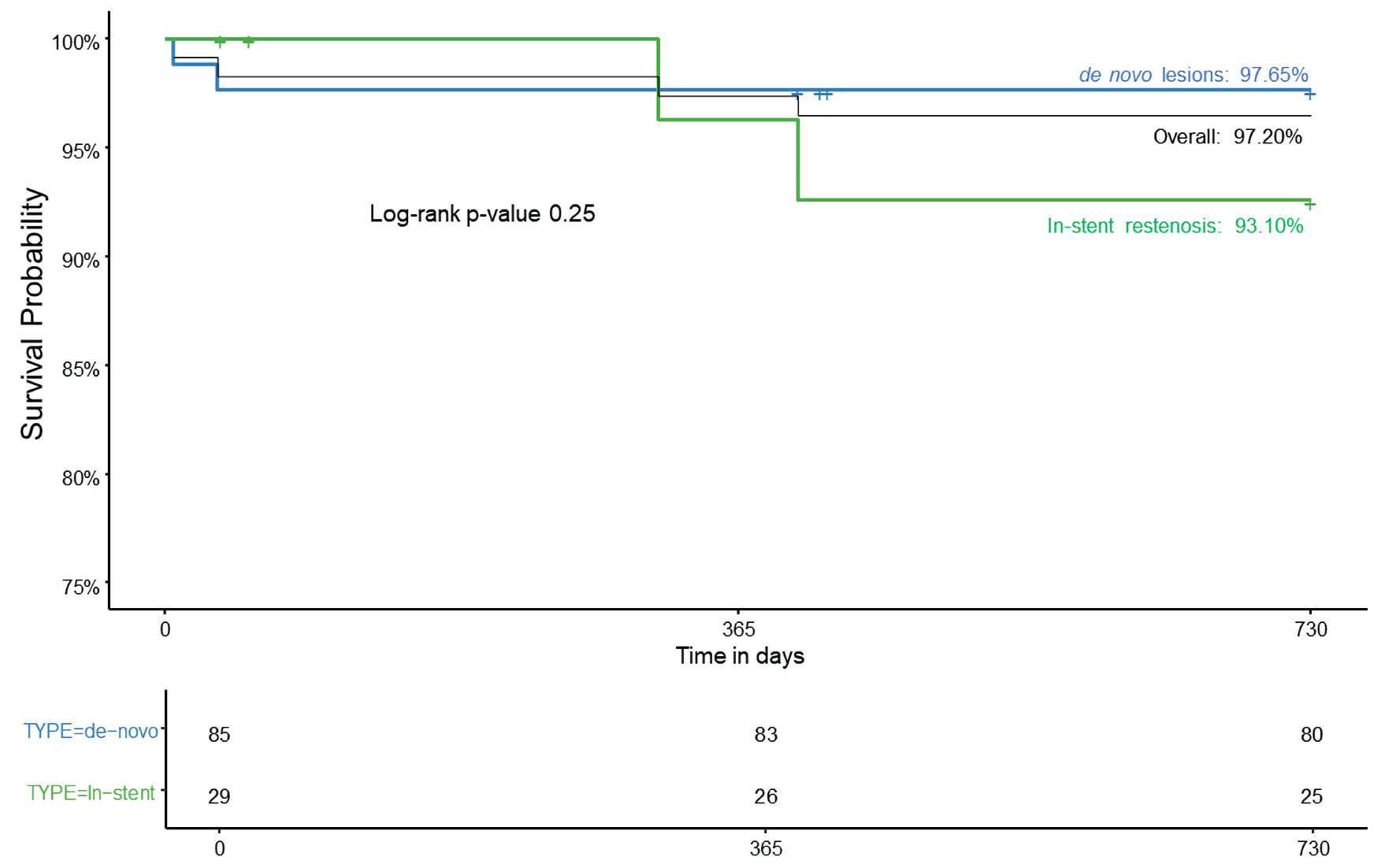 Click for large image | Figure 3. Kaplan-Meier curve for overall survival rate at 24-month follow-up. |
Late lumen loss
QCA analysis showed significant improvement in hemodynamic parameters at the 6-month follow-up. The analysis of LLL using QCA is detailed in Table 4 and visually represented in Figure 4.
 Click to view | Table 4. Quantitative Coronary Angiographic Analysis |
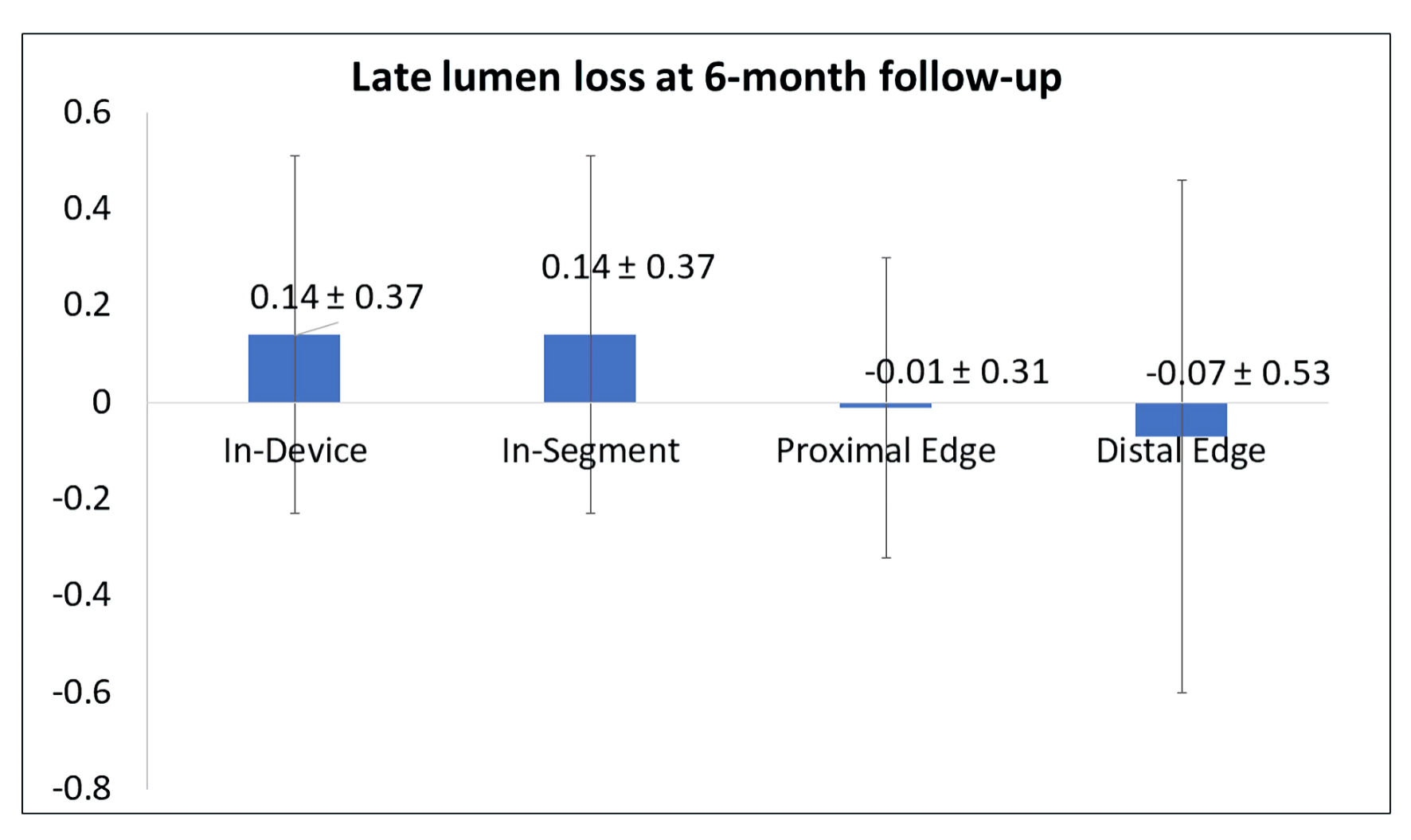 Click for large image | Figure 4. The late lumen loss at 6-month follow-up. |
User rating on technical properties
The device received good user rating scores across a range of critical parameters, as evaluated by operators. The flexibility rates received a score of 4.41 ± 0.68, showcasing its adaptability to various anatomical structures. Pushability and trackability scored 4.55 ± 0.57 and 4.55 ± 0.51, respectively, allowing smooth navigation through tortuous vessels. Crossability achieved a rating of 4.43 ± 0.66, efficiently crossing lesions with minimal resistance. Inflation and deflation times were swift, with ratings of 4.59 ± 0.52 and 4.55 ± 0.53, respectively, contributing to efficient positioning and retraction during procedures. Radiopaque marker visibility rated 4.62 ± 0.53, ensuring accurate placement under fluoroscopy. The ease of removal scored 4.68 ± 0.51, facilitating streamlined retrieval post-procedure, thus enhancing overall procedural efficiency and patient comfort.
| Discussion | ▴Top |
This study investigated the safety and effectiveness of the MOZEC sirolimus-eluting PTCA balloon dilatation catheter for managing several coronary artery conditions in a wide range of clinical scenarios. This study presented noteworthy clinical and angiographic results. MACEs occurred at a rate of 4.47%, while all-cause mortality and TLR rates were 2.99%. Angiographic outcomes revealed significant improvement in the minimal lumen diameter (MLD) both in-device and in-segment. The in-device MLD at baseline was 0.49 ± 0.37 mm, which increased to 1.15 ± 0.47 mm at the 6-month follow-up (P = 0.0005). Similarly, the in-segment MLD improved from 0.49 ± 0.37 mm baseline to 1.15 ± 0.47 mm at 6 months (P = 0.0005). These findings indicated substantial improvements in luminal dimensions from baseline to 6 months and have demonstrated a favorable safety and efficacy profile of MOZEC SEB PTCA balloon.
The follow-up duration of the current investigation is notably longer than that of previously published studies, which typically reported outcomes at 1 year following the application of drug-coated balloon (DCB), such as the EASTBOURNE registry (an investigator-initiated study that enrolled real-world patients). The primary endpoint was TLR at 12 months, which was observed at a rate of 5.90%. Additional outcomes at 12 months included a MACE rate of 9.90%, an all-cause mortality rate of 2.50%, and a cardiac mortality rate of 1.50%. Comparing these findings to the current investigation, TLR rates were notably lower at the 24-month follow up, while MACE and all-cause mortality rates remained comparable [17]. The longer-term follow-up in the current study provided a comprehensive understanding of the sustained efficacy and safety of DCBs in clinical practice [18].
The Nanolute registry, a prospective study designed to evaluate the clinical performance of another sirolimus-coated balloon (SCB), focused on the treatment of de novo coronary lesions and ISR. At 24-month follow-up, the registry reported a MACE rate of 4.20%, MI rate of 0.20%, an all-cause mortality rate of 1.70%, a cardiac mortality rate of 0.7%, and TLR rate of 3.20% [19]. The current investigation has also reported outcomes comparable to those observed in the Nanolute registry, further supporting the clinical performance and effectiveness of SCBs in managing coronary artery disease. The incidence of MACE was relatively low (4.47%) and comparable to those observed in studies involving small vessels [12, 14].
Moreover, MOZEC SEB demonstrated clinical outcomes comparable to other SEBs with higher drug concentrations (4 µg/mm2) and crystalline designs. Notably, the rate of MACEs observed in this study was lower than that seen in large clinical trials for SEB [12]. Previous studies have highlighted the favorable vessel response after DCB application in chronic total occlusions (CTOs) [16]. Building on this foundation, our study performed angiographic assessments of DCB application at mid-term follow-up, revealing favorable outcomes at 6 months, specifically regarding vessel remodeling. These findings align with data observed for other SEBs [20].
The treatment of ISR with sirolimus DEBs has shown varied outcomes regarding mortality and MACEs. While some studies indicate a favorable safety profile, others highlight significant occurrences of MACEs in patients treated with these devices [21-23]. ISR CTOs represented 15% of all CTOs in percutaneous interventions and were associated with higher long-term MACE rates compared to de novo CTOs, despite similar procedural success [24]. Similar to this, our study had more rate of mortality and MACEs in the ISR group.
Table 5 [14, 17, 19, 21, 25-29] shows the comparison of clinical outcomes of MOZEC SCB to other contemporary DCB (SeQuent Please, and MagicTouch) and DES (XIENCE, Resolute, Resolute Onyx, MiStent, Endeavor Sprint, Biolimus, and Orsiro). Notably, the MOZEC SEB demonstrates a favorable safety profile with relatively low incidences of MACEs (4.47%), cardiac death (1.49%), MI (3.73%) and TLR (2.99%) compared to other DES, which reported higher rates of adverse events. This comparison underscores the competitive efficacy of the MOZEC SEB in reducing MACEs and improving patient outcomes [25-29].
 Click to view | Table 5. Comparison of 2-Year Outcomes of MOZEC SEB With Contemporary DCB and DES |
The results of this study, in conjunction with previously published outcomes of SEB application in de novo lesions, have significant implications for redefining the approach to coronary artery disease intervention. Given that DCB application has shown favorable clinical outcomes across different clinical scenarios, the choice between DESs and DEBs in specific clinical scenarios or lesion morphologies warrants careful consideration [30]. Furthermore, high-bleeding risk patients may benefit from DEB application due to shortened DAPT requirements [14].
Study limitations
The study has several limitations. Despite the inherent limitations of a single-arm study design, the observed outcomes provide a robust rationale for validation through future randomized controlled trials. Beyond the study design, there was no angiographic follow-up for every participant, although the low MACE rates suggest positive clinical outcomes. Additionally, the study population was heterogeneous, encompassing patients across a variety of clinical scenarios. However, previous large registries on SEB application in de novo lesions have indicated superior clinical outcomes compared to its application in ISR. Furthermore, the study represents a mid-term follow-up; thus, longer-term observations are necessary to fully ascertain the safety and efficacy of the MOZEC SEB application. Lastly, admission diagnoses, specifying whether conditions were acute coronary syndromes, stable, or others, were not recorded.
Conclusions
The application of MOZEC SEB in various clinical scenarios demonstrates safety and efficacy over the long-term 24-month follow-up. These findings align with the favorable vessel healing observed at the 6-month imaging follow-up.
| Supplementary Material | ▴Top |
Suppl 1. Study schedule and assessments.
Acknowledgments
None to declare.
Financial Disclosure
This research was funded by Meril Life Sciences Pvt. Ltd., India.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Informed Consent
All patients signed the informed consent.
Author Contributions
RKPJ and KS: conceptualization, and formal analysis. RKPJ and KP: methodology. All authors: investigation, resources, data curation, writing - original draft preparation; writing - review and editing, visualization, funding acquisition. RKPJ, KP and KS: supervision. All authors have read and agreed to the published version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ACC: American College of Cardiology; ACS: acute coronary syndrome; AHA: American Heart Association; CAD: coronary artery disease; DAPT: dual antiplatelet therapy; DEBs: drug-eluting balloons; DESs: drug-eluting stents; LLL: late lumen loss; MACEs: major adverse cardiac events; MI: myocardial infarction; PCI: percutaneous coronary intervention; PMS: post-marketing surveillance; PTCA: percutaneous transluminal coronary angioplasty; QCA: quantitative coronary angiography; RBP: rated burst pressure; SEB: sirolimus-eluting balloon; SPSS: Statistical Package for the Social Sciences; TIMI: Thrombolysis in Myocardial Infarction; TLR: target lesion revascularization
| References | ▴Top |
- Ralapanawa U, Sivakanesan R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: a narrative review. J Epidemiol Glob Health. 2021;11(2):169-177.
doi pubmed - Brugaletta S, Gomez-Lara J, Ortega-Paz L, Jimenez-Diaz V, Jimenez M, Jimenez-Quevedo P, Diletti R, et al. 10-year follow-up of patients with Everolimus-Eluting versus Bare-Metal stents after ST-Segment elevation myocardial infarction. J Am Coll Cardiol. 2021;77(9):1165-1178.
doi pubmed - Zheng Y, Li J, Wang L, Yu P, Shi H, Wu L, Chen J. Effect of Drug-coated balloon in side branch protection for de novo coronary bifurcation lesions: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:758560.
doi pubmed - Cortese B, Testa G, Rivero F, Erriquez A, Alfonso F. Long-term outcome of drug-coated balloon vs drug-eluting stent for small coronary vessels: PICCOLETO-II 3-year follow-up. JACC Cardiovasc Interv. 2023;16(9):1054-1061.
doi pubmed - Latib A, Agostoni P, Dens J, Patterson M, Lancellotti P, Tam FCC, Schotborgh C, et al. Paclitaxel drug-coated balloon for the treatment of de novo small-vessel and restenotic coronary artery lesions: 12-month results of the prospective, multicenter, single-arm PREVAIL study. J Invasive Cardiol. 2021;33(11):E863-E869.
doi pubmed - Kondo Y, Ishikawa T, Shimura M, Yamada K, Ukaji T, Tamura Y, Arai M, et al. Cardiovascular outcomes after paclitaxel-coated balloon angioplasty versus drug-eluting stent placement for acute coronary syndrome: a systematic review and meta-analysis. J Clin Med. 2024;13(5):1481.
doi pubmed - Vos NS, Fagel ND, Amoroso G, Herrman JR, Patterson MS, Piers LH, van der Schaaf RJ, et al. Paclitaxel-coated balloon angioplasty versus drug-eluting stent in acute myocardial infarction: the REVELATION randomized trial. JACC Cardiovasc Interv. 2019;12(17):1691-1699.
doi pubmed - Januszek R, Bil J, Gilis-Malinowska N, Staszczak B, Figatowski T, Milewski M, Mielczarek M, et al. Long-term outcomes following drug-eluting balloon or thin-strut drug-eluting stents for treatment of in-stent restenosis stratified by duration of dual antiplatelet therapy (DEB-Dragon Registry). Postepy Kardiol Interwencyjnej. 2022;18(1):14-26.
doi pubmed - Tesfamariam B. Local arterial wall drug delivery using balloon catheter system. J Control Release. 2016;238:149-156.
doi pubmed - Yamamoto T, Sawada T, Uzu K, Takaya T, Kawai H, Yasaka Y. Possible mechanism of late lumen enlargement after treatment for de novo coronary lesions with drug-coated balloon. Int J Cardiol. 2020;321:30-37.
doi pubmed - Kawai T, Watanabe T, Yamada T, Morita T, Furukawa Y, Tamaki S, Kawasaki M, et al. Coronary vasomotion after treatment with drug-coated balloons or drug-eluting stents: a prospective, open-label, single-centre randomised trial. EuroIntervention. 2022;18(2):e140-e148.
doi pubmed - Cortese B, Di Palma G, Guimaraes MG, Piraino D, Orrego PS, Buccheri D, Rivero F, et al. Drug-coated balloon versus drug-eluting stent for small coronary vessel disease: PICCOLETO II randomized clinical trial. JACC Cardiovasc Interv. 2020;13(24):2840-2849.
doi pubmed - Zhang Y, Zhang X, Dong Q, Chen D, Xu Y, Jiang J. Duration of dual antiplatelet therapy after implantation of drug-coated balloon. Front Cardiovasc Med. 2021;8:762391.
doi pubmed - Jeger RV, Farah A, Ohlow MA, Mangner N, Mobius-Winkler S, Leibundgut G, Weilenmann D, et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet. 2018;392(10150):849-856.
doi pubmed - Hu FW, Chang S, Li Q, Zhu YX, Wang XY, Cheng YW, Zhou QH, et al. Long-term clinical outcomes after percutaneous coronary intervention with drug-coated balloon-only strategy in de novo lesions of large coronary arteries. Front Cardiovasc Med. 2022;9:882303.
doi pubmed - Qin Q, Chen L, Ge L, Qian J, Ma J, Ge J. Long-term clinical outcomes of drug-coated balloon for the management of chronic total occlusions. Coron Artery Dis. 2023;34(8):555-561.
doi pubmed - Cortese B, Testa L, Heang TM, Ielasi A, Bossi I, Latini RA, Lee CY, et al. Sirolimus-coated balloon in an all-comer population of coronary artery disease patients: the EASTBOURNE prospective registry. JACC Cardiovasc Interv. 2023;16(14):1794-1803.
doi pubmed - Wang D, Wang X, Yang T, Tian H, Su Y, Wang Q. Drug-coated balloons for de novo coronary artery lesions: a meta-analysis of randomized clinical trials. Yonsei Med J. 2023;64(10):593-603.
doi pubmed - El-Mokdad R, di Palma G, Cortese B. Long-term follow-up after sirolimus-coated balloon use for coronary artery disease. Final results of the Nanolute study. Catheter Cardiovasc Interv. 2020;96(5):E496-E500.
doi pubmed - Ahmad WAW, Nuruddin AA, Abdul Kader M, Ong TK, Liew HB, Ali RM, Mahmood Zuhdi AS, et al. Treatment of coronary de novo lesions by a sirolimus- or paclitaxel-coated balloon. JACC Cardiovasc Interv. 2022;15(7):770-779.
doi pubmed - Caiazzo G, De Michele M, Golino L, Manganiello V, Fattore L. Sirolimus-eluting balloon for the treatment of coronary lesions in complex ACS patients: the SELFIE registry. J Interv Cardiol. 2020;2020:8865223.
doi pubmed - Kheifets M, Rahat O, Bental T, Levi A, Vaknin-Assa H, Greenberg G, Codner P, et al. Outcomes of drug-eluting balloons for in-stent restenosis: large cohort analysis and single-center clinical experience. Can J Cardiol. 2024;40(7):1250-1257.
doi pubmed - Verheye S, Vrolix M, Kumsars I, Erglis A, Sondore D, Agostoni P, Cornelis K, et al. The SABRE trial (Sirolimus Angioplasty Balloon for Coronary In-Stent Restenosis): angiographic results and 1-year clinical outcomes. JACC Cardiovasc Interv. 2017;10(20):2029-2037.
doi pubmed - Vemmou E, Quadros AS, Dens JA, Rafeh NA, Agostoni P, Alaswad K, Avran A, et al. In-Stent CTO percutaneous coronary intervention: individual patient data pooled analysis of 4 multicenter registries. JACC Cardiovasc Interv. 2021;14(12):1308-1319.
doi pubmed - Buiten RA, Ploumen EH, Zocca P, Doggen CJM, Jessurun GAJ, Schotborgh CE, Roguin A, et al. Thin Composite-Wire-Strut Zotarolimus-Eluting stents versus Ultrathin-Strut Sirolimus-Eluting stents in BIONYX at 2 years. JACC Cardiovasc Interv. 2020;13(9):1100-1109.
doi pubmed - Kiatchoosakun S, Pienvichit P, Kuanprasert S, Suraphakdee N, Phromminikul A. A clinical evaluation of the XIENCE V everolimus eluting stent in the treatment of patients with coronary artery disease: Result from Thailand Registry - XIENCE V performance evaluation (THRIVE study). Indian Heart J. 2017;69(2):165-169.
doi pubmed - Meredith IT, Worthley SG, Whitbourn R, Walters D, McClean D, Ormiston J, Horrigan M, et al. Long-term clinical outcomes with the next-generation Resolute Stent System: a report of the two-year follow-up from the RESOLUTE clinical trial. EuroIntervention. 2010;5(6):692-697.
doi pubmed - Wijns W, Suttorp MJ, Zagozdzon L, Morice MC, McClean D, Stella P, Donohoe D, et al. Evaluation of a crystalline sirolimus-eluting coronary stent with a bioabsorbable polymer designed for rapid dissolution: two-year outcomes from the DESSOLVE I and II trials. EuroIntervention. 2016;12(3):352-355.
doi pubmed - Raber L, Kelbaek H, Ostojic M, Baumbach A, Heg D, Tuller D, von Birgelen C, et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA. 2012;308(8):777-787.
doi pubmed - van Veelen A, Kucuk IT, Fuentes FH, Kahsay Y, Garcia-Garcia HM, Delewi R, Beijk MAM, et al. First-in-human drug-eluting balloon treatment of vulnerable lipid-rich plaques: rationale and design of the DEBuT-LRP study. J Clin Med. 2023;12(18):5807.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.