| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 2, April 2025, pages 153-160
The Prognostic Value of Circulating Cytokines and Complete Blood Count-Based Inflammatory Markers in COVID-19 Patients With Atrial Fibrillation
Giorgi Tcholadzea, e, Ia Pantsulaiaa, b, e, Levan Ratianic, Lela Kopaleishvilid, Tamar Bolotashvilid, Avtandil Jorbenadzed, Tinatin Chikovania
aDepartment of Immunology, Tbilisi State Medical University, Tbilisi 0177, Georgia
bVl. Bakhutashvili Institute of Medical Biotechnology, Tbilisi State Medical University, Tbilisi 0159, Georgia
cTSMU First University Clinic, Tbilisi 0141, Georgia
dChapidze Heart Disease Center, Tbilisi 0159, Georgia
eCorresponding Author: Giorgi Tcholadze and Ia Pantsulaia, Department of Immunology, Tbilisi State Medical University, Tbilisi 0177, Georgiaand
Manuscript submitted December 6, 2024, accepted February 5, 2025, published online February 20, 2025
Short title: Clinical Markers and the Severity of COVID-19 With AF
doi: https://doi.org/10.14740/cr2027
| Abstract | ▴Top |
Background: Atrial fibrillation (AF) is associated with a high burden of cardiovascular disease, which has been worsened during the coronavirus disease 2019 (COVID-19) pandemic. The purpose of this study was to assess the association between clinical markers, especially interleukin-6 (IL-6) and other inflammatory biomarkers, and the severity of COVID-19 in patients with AF.
Methods: This retrospective cohort study categorized patients based on clinical presentations and laboratory results to investigate the prognostic significance of inflammatory markers in COVID-19 outcomes among those with AF. The study included 100 hospitalized COVID-19 patients aged between 40 to 80 years and was conducted at the Chapidze Hospital in Tbilisi, Georgia. Patients were then grouped by disease severity according to computed tomography (CT) scores, clinical symptoms, respiratory rate and oxygen saturation. Levels of IL-6 were obtained at three time points during hospitalization. A broad range of laboratory tests, including C-reactive protein (CRP), ferritin, and D-dimer, were also conducted.
Results: Patients with AF demonstrated significantly elevated levels of IL-6 (P = 0.024), CRP (P = 0.001), and ferritin (P < 0.001), suggesting a severe inflammatory response. D-dimer levels were also notably higher in the AF group (P < 0.005), indicating an increased risk of thrombotic complications. Oxygen saturation levels were significantly lower (P = 0.004) and CT scores higher in patients with AF. Furthermore, the length of hospitalization was longer among patients with AF (median duration significantly higher, P = 0.032), indicating a more severe disease course.
Conclusions: The proinflammatory markers such as IL-6 are independent predictive markers of COVID-19 severity in AF patients. Overall, it highlights urgent treatment approaches, such as available anti-inflammatory drugs, for COVID-19 patients with arrhythmias. Combining these biomarkers into clinical routines helps us better identify patients at risk and how to treat them.
Keywords: COVID-19; Atrial fibrillation; IL-6; Inflammatory markers; Cytokines
| Introduction | ▴Top |
The coronavirus disease 2019 (COVID-19) pandemic has affected more than 670 million people and has caused roughly 7 million deaths worldwide as of January 2023 [1]. Chronic COVID-19, characterized by lingering symptoms or delayed/long-term complications, is becoming increasingly well-documented. Among cardiovascular (CV) risk factors and morbidity, disease severity was found to be associated with these early in the pandemic. COVID-19 poses a plethora of significant risks to those with pre-existing CV disease, as the World Health Organization recently noted an increased mortality rate and more severe disease progression in these patients [2, 3]. One in five of COVID-19 hospitalized patients present with elevated troponin, suggestive of myocardial injury. Myocardial injury (21.2%) was independently associated with higher mortality, worse outcomes and an increased need for intensive care unit (ICU) admission and was an independent predictor of death. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can cause direct myocardial damage, leading to myocarditis, or indirect damage through myocardial infarction due to hypoxemia [2, 4].
Cardiac arrhythmias have emerged as initial clinical manifestations of COVID-19, with incidences reported between 10.11% and 15.3% [5]. Atrial arrhythmia was noted in 9.2% of cases. Patients with new arrhythmias are at a heightened risk of severe disease and ICU admission. In ICU settings, the incidence of cardiac arrhythmia reached 44.4%, with atrial fibrillation (AF), non- sustained ventricular tachycardia (NSVT), and cardiac arrest being prevalent. COVID-19 survivors face an increased risk of arrhythmia-related disorders, including AF and atrial flutter. This underscores the evolving understanding of tachycardia and COVID-19, highlighting the need for further research to inform management strategies [6]. Given the improbability of SARS-CoV-2 directly causing cytotoxic effects on cardiac myocytes, the detrimental effects are more likely due to the hyperinflammatory response of the innate immune system [7].
Previous studies have shown that interleukins (ILs) like IL-6 and IL-1 can extend the duration of the ventricular action potential by altering the expression or function of various ion channels in cardiomyocytes, particularly potassium (K+) and calcium (Ca2+) channels [8, 9]. These alterations are known as inflammatory cardiac channelopathies. Furthermore, several growth factors, such as transforming growth factor-beta (TGF-β), platelet-derived growth factors (PDGF), and cytokines (including tumor necrosis factor-alpha (TNF-α), IL-1, IL-6, IL-10, and IL-4), along with neurohumoral pathways, can promote myocardial fibrosis by initiating signaling cascades via surface receptor binding, which then activates downstream signaling pathways [10, 11]. The cytokine storm often associated with severe SARS-CoV-2 infections can induce structural remodeling by activating fibrosis pathways. Additionally, due to molecular mimicry, an autoimmune response against cardiac antigens might contribute to cardiac damage in post-COVID patients. Anti-cardiac antibodies are part of the broader systemic immune and inflammatory response to SARS-CoV-2 [12, 13]. Thus, investigating clinical markers indicative of the severity of COVID-19, researchers are exploring various factors that can predict the progression of the disease [14]. Thus, the goal of this study was to assess the relationship between inflammatory markers (IL-6, inflammatory indices), AF, and severity of COVID-19 in Georgian population.
| Materials and Methods | ▴Top |
The study was conducted at the Chapidze Heart Hospital, Tbilisi, Georgia. The study included 100 patients aged 40 to 80 years who had symptoms of COVID-19, and reverse transcription polymerase chain reaction (RT-PCR) confirmed the diagnosis. The patient’s inclusion criteria were CV disease, laboratory-confirmed COVID-19 infection and hospitalization. Disease severity was classified according to symptoms, respiratory rate, oxygen saturation, and computed tomography (CT) scores, which indicated lung damage. The Ethics Commission of Tbilisi State Medical University approved the study (N 1-2021/85), and the study complies with the Declaration of Helsinki. Informed consent was obtained from all patients. A legally certified bilateral agreement was established between Tbilisi State Medical University and Chapidze Hospital.
Cytokine and other clinical laboratory parameters
Serum IL-6 was determined using an electrochemiluminescence immunoassay with a Roche Cobas e411 (Hoffmann-La Roche Ltd, Switzerland). The lower detection limit for IL-6 was 1.5 pg/mL, and the upper limit was 5,000 pg/mL without pre-dilution. IL-6 levels were determined at least three times: 1) at hospitalization; 2) within 1 week after hospitalization; and 3) before discharge from the hospital. A comprehensive panel of laboratory tests was performed, including alanine aminotransferase, aspartate aminotransferase, creatinine, lactate dehydrogenase, prothrombin time, prothrombin index (PI), international normalized ratio, activated partial thromboplastin time, fibrinogen concentration, D-dimer, troponin, C-reactive protein (CRP), procalcitonin, ferritin, complete blood count (CBC), and IL-6. These tests were conducted using advanced equipment such as the Roche Cobas e411 (Hoffmann-La Roche Ltd, Switzerland), ensuring the accuracy and reliability of our data.
Statistical analysis
Statistical calculations were performed using STATISTICA (Statsoft, Inc., USA). The statistical analysis strategy was divided into different stages: 1) Preliminary descriptive statistics (mean ± standard deviation (SD)); 2) The patients were scanned according to gender and age; 3) The analysis of variance (ANOVA) analysis was used to compare the variation of studied parameters between and within these groups. Patients were divided into two groups to reveal correlations of disease severity and potential biomarkers: with and without arrhythmic conditions (1 = arrhythmia, 2 = without arrhythmia). The Kolmogorov-Smirnov and Lilliefors tests for normality were used to assess the normal distribution of the cytokines. Since the distributions of the studied molecules were markedly skewed, the data on biochemical markers were log-transformed to correct for non-normality before further analysis. A review of the distribution properties characterizing the studied molecules showed some values to be outliers (≥ 4 SD), which were not included in further analysis. Differences in means of the plasma cytokines between male and female groups were determined by the Mann-Whitney U test. A two-way repeated measures ANOVA was used at specific time points to evaluate differences between the groups. A P value of 0.05 or less was deemed significant for all analyses.
Patient management
Ventricular rate control for patients with AF was guided by established European Heart Rhythm Association (EHRA) guidelines. Beta-blockers and/or calcium channel blockers were used as first-line agents. In patients with heart failure or those unable to tolerate first-line agents, digoxin was administered for rate control.
| Results | ▴Top |
Inflammatory markers and clinical parameters of the patients grouped according to the presence or absence of AF are shown in Table 1.
 Click to view | Table 1. Comparison of Inflammatory Markers and Clinical Parameters Between Patients With and Without Atrial Fibrillation (AF) |
Cytokines and clinical markers data are shown before log transformation in their original units. At the first stage of analysis, concentrations of the investigated cytokine (IL-6) and clinical parameters (troponin, D-dimer, procalcitonin, creatinine, lactate dehydrogenase, prothrombin time, PI, activated partial thromboplastin time, fibrinogen concentration, CRP, procalcitonin, ferritin, blood cells) were scanned by gender and age. The comparison of mean levels showed no significant gender dependency. Since the values for male and female were not significantly different, statistical analysis was continued for combined data. Based on the statistical analysis, patients with arrhythmic conditions have a significant time-dependent increase in CRP compared to patients without arrhythmias. More specifically, the second and third measurements (CRP-2 and CRP-3) were significantly different (P = 0.044 and P = 0.001, respectively). This indicates that inflammation, as measured by CRP, has a greater effect on COVID-19 disease course in patients with arrhythmic disorders. Ferritin levels increased significantly, even more pronounced in patients with arrhythmias in the third measurement (FERIT-3) (P < 0.001) (Fig. 1). This could imply a link between iron homeostasis disturbances and the severity of COVID-19 in the context of cardiac arrhythmia.
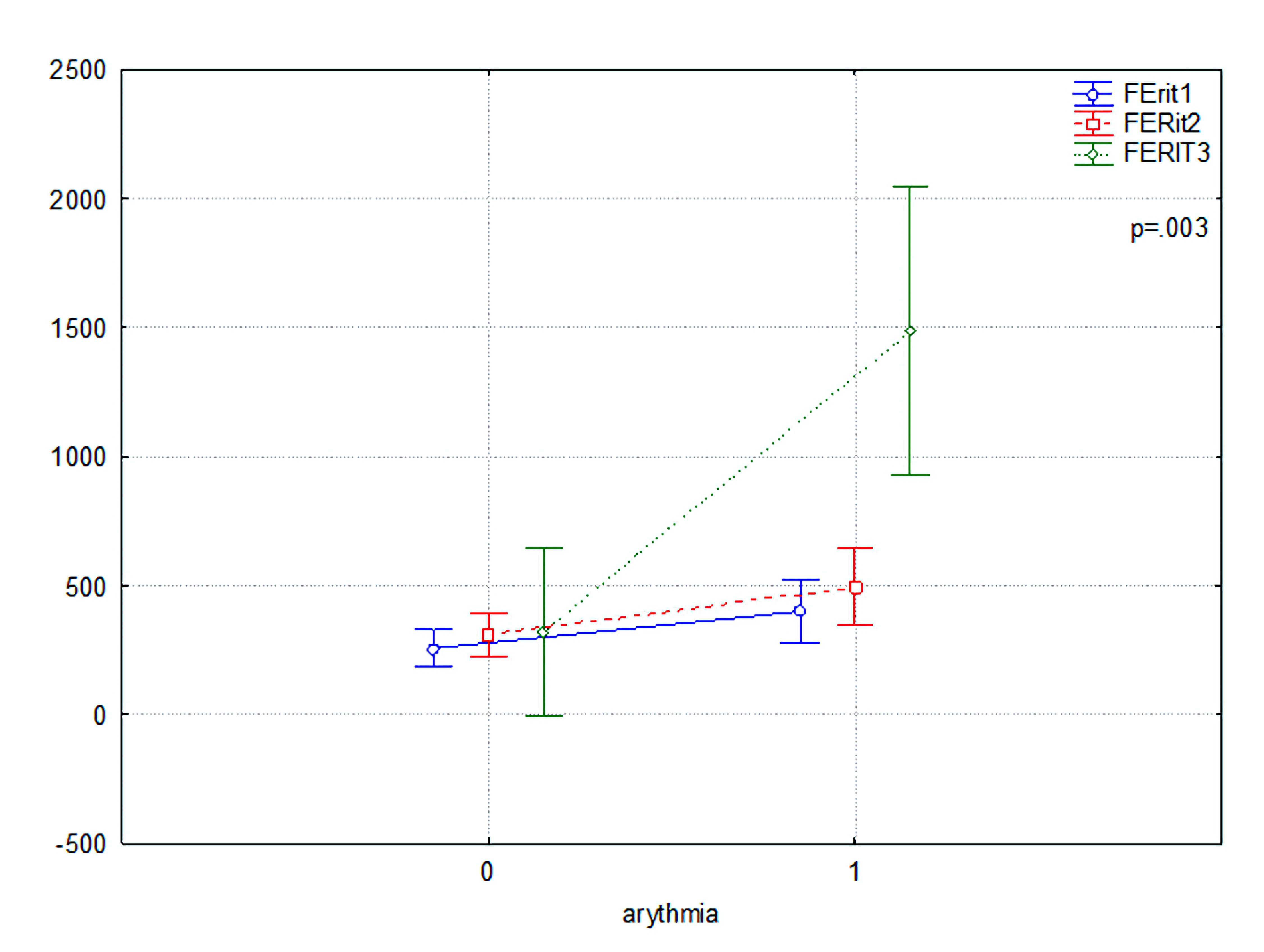 Click for large image | Figure 1. Comparison of ferritin (FERIT1, FERIT2, and FERIT3) levels in patients with (1) and without (0) atrial fibrillation. |
There were very significant differences in the levels of D-dimer with the first, second, and third measurements (Dimer1, Dimer2, Dimer3) in the D-dimer levels (P < 0.005) (Fig. 2), with levels being higher in patients with arrhythmias. We concluded that this group has so far demonstrated an increased incidence of thromboembolic complications.
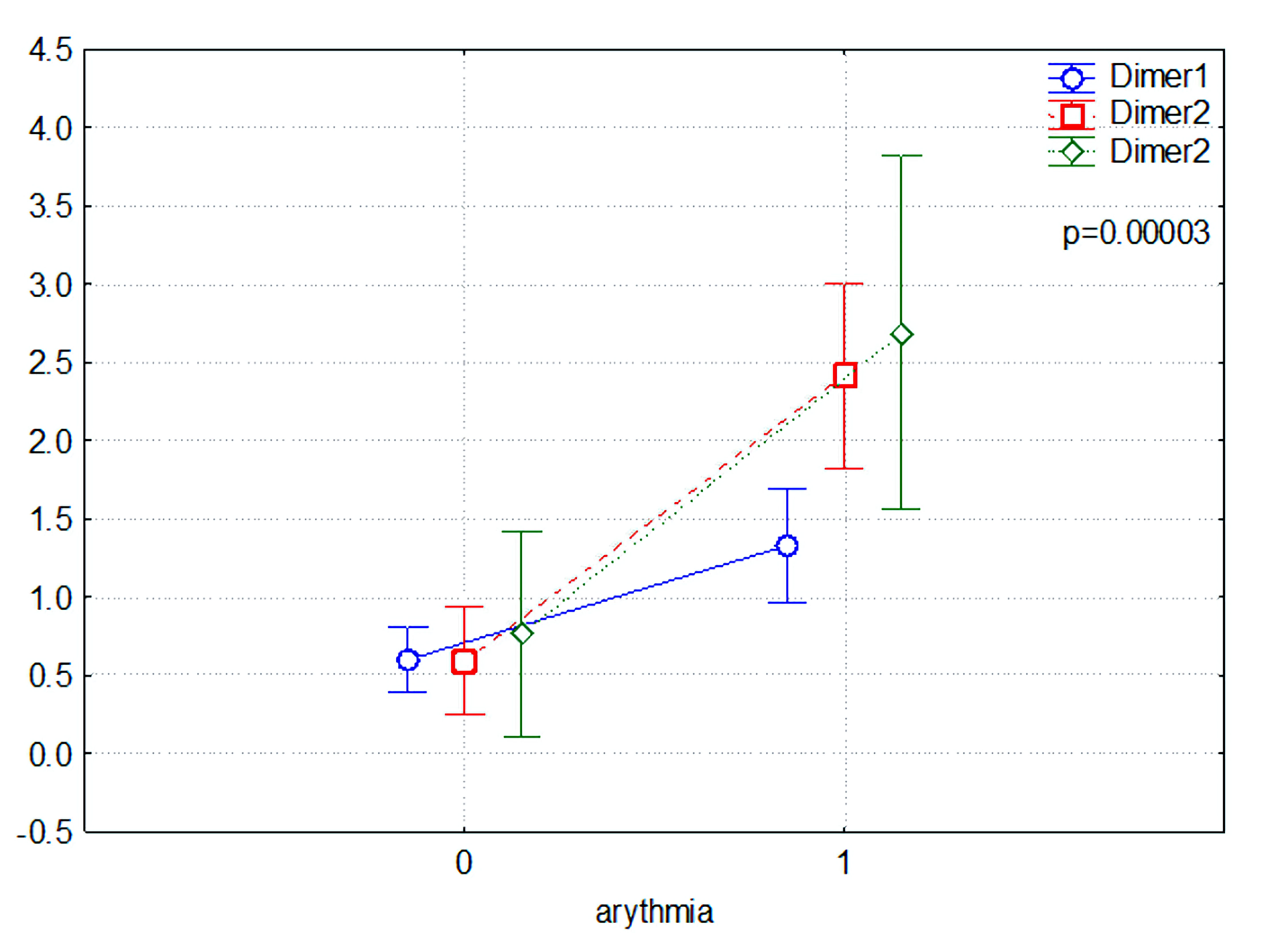 Click for large image | Figure 2. Comparison of D-dimer levels between patients with (1) and without (0) atrial fibrillation. |
The first (saturation1) and second (saturation2) time points for oxygen saturations were significantly lower in arrhythmic patients (P = 0.010 and P = 0.004, respectively), highlighting the potential consequences of arrhythmic conditions in COVID-19 respiratory function. In addition, the median hospitalization duration was significantly longer for arrhythmic patients, with a more comprehensive interquartile range and outliers indicating prolonged stays in some cases. Figure 3 illustrates the changes in IL-6 serum levels among COVID-19 patients with AF (1) and without AF (0). The IL-6 levels are represented at three different time points: IL-6(1), IL-6(2), and IL-6(3). Statistical analysis reveals a significant difference between the groups (P = 0.024), underscoring the heightened inflammatory response associated with AF in COVID-19 patients. These findings align with the elevated IL-6 levels, showing an increased disease burden in patients with cardiac arrhythmias. Several markers, including the neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), erythrocyte sedimentation rate (ESR), liver enzymes (ALT, AST), and kidney function (creatinine), showed no statistically significant differences between the groups, indicating that these parameters may not be as crucial in distinguishing the severity of COVID-19 in patients with and without arrhythmic conditions.
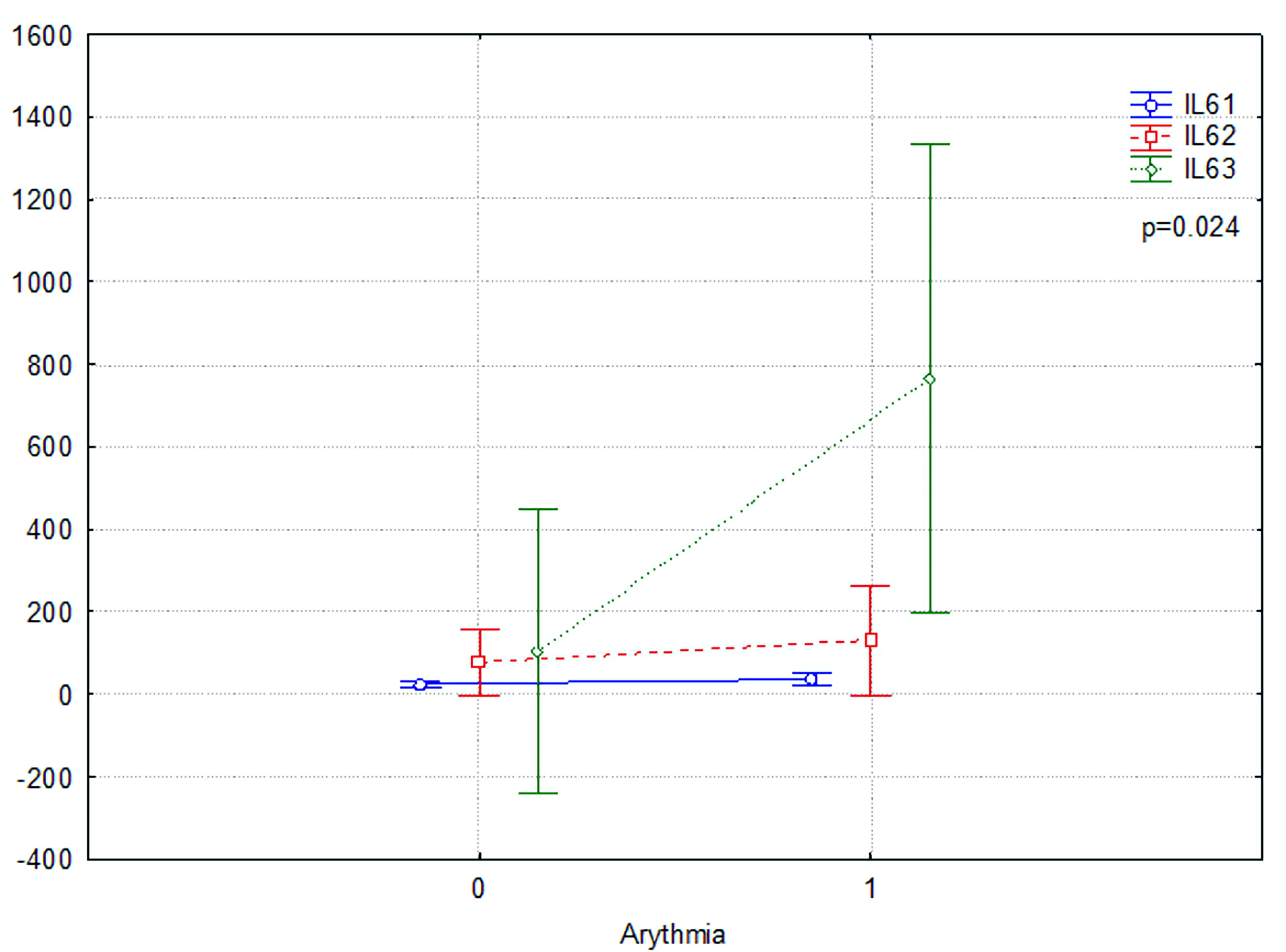 Click for large image | Figure 3. IL-6 serum levels change in COVID-19 patients with (1) or without atrial fibrillation (0) group. IL: interleukin; COVID-19: coronavirus disease 2019. |
Spontaneous conversion
Among patients with paroxysmal AF, a subset spontaneously converted to sinus rhythm during the recovery phase of COVID-19, particularly those with lower inflammatory marker levels and less structural heart damage. In contrast, patients with persistent AF often required aggressive interventions for rhythm management.
Persistent AF post-COVID
During follow-up after discharge, 12 patients were identified with persistent AF, highlighting a significant burden of sustained arrhythmias in the post-COVID phase.
| Discussion | ▴Top |
Our results provide indirect evidence that COVID-19 severity is influenced by different inflammatory and thrombotic pathways in patients with cardiac arrhythmias, offering valuable insight into the global impact of such underlying conditions on risk status and their management implications.
Increased serum levels of CRP and ferritin in patients with arrhythmias tend to indicate an exacerbated systemic inflammation response and are associated with systemic inflammation influencing poor outcome in prior studies [15-17]. Likewise, elevated D-dimer levels, as have previously been described in literature, further emphasize the association with thrombotic events and increased risk in COVID-19 patients, especially those with cardiovascular complications [18-20]. The crucial distinction in leukocytes counts and the platelet-to-lymphocyte ratio (PLR) additionally highlight the relationship between inflammation and severity of coronavirus infection in patients with arrhythmias. These markers may be related to a longitudinal disease process and thus may represent useful outcome measures for tracking the evolution of the disease and for guiding interventions in this cohort. In contrast, the lack of meaningful differences in NLR, SII, and landmark metabolic variables (e.g., liver enzymes and creatinine) implies that not all inflammatory or metabolic disturbances have the same predictive ability with regard to COVID-19 severity in terms of arrhythmias [21, 22], highlighting the need for a multifactorial approach in the assessment and management of these patients. Finally, the more accentuated hypoxemia in the arrhythmic patients only adds to the need for respiratory monitoring and support in the management of COVID-19 [23, 24]. This insight may represent the cumulative impact of CV and pulmonary involvement in the pathophysiology of the disease. However, the retrospective design of the study and the presence of possible confounding factors not taken into account in the analysis should be considered. Prospective studies are warranted to validate these dose-response associations and to elucidate the mechanisms underpinning these associations. Studying the effect of certain arrhythmias on the outcome of COVID-19 might give more refined results in terms of management of the patients too.
Similar to our observations, multiple recently published analyses have drawn attention to the importance of inflammatory and thrombotic pathways in arrhythmia-free patients with COVID-19. A study by Garcia et al (2003), for instance further supporting our observations of augmented inflammation, found that increased CRP and ferritin levels were highly predictive for adverse clinical outcome in patients with pre-existing arrhythmias experiencing COVID-19 [25]. Likewise, Lee et al [26] found that D-dimer levels were considerably elevated among arrhythmic patients with severe COVID-19, suggesting a relationship between these aforementioned arrhythmic complications and hypercoagulability. Taken together with our own studies, these findings suggest that inflammatory and thrombotic biomarkers could be valuable biomarkers of disease development and potential aids in treatment management in this high-risk population. Additionally, although no statistically significant association was found in our study with markers like the NLR, other studies have shown different results. Consequently, what is regarded as a good prognostic biomarker may vary according to the patient population, as suggested by Salvatore et al [27], who found that the NLR was associated with adverse outcomes in COVID-19 patients with CV comorbidities. In line with our findings on the prognostic value of the NLR in COVID-19, several other studies have similarly identified NLR as an independent prognostic indicator of in-hospital mortality among COVID-19 patients. For instance, a study by Liu et al [28] demonstrated a significant association between elevated NLR values and increased mortality rates. Similarly, research by Yang et al [29], and more recently, Zeng et al [30], have corroborated these findings, further emphasizing the utility of NLR as a reliable biomarker for assessing disease severity and mortality risk in hospitalized COVID-19 patients. These studies underscore the importance of incorporating NLR into clinical assessment protocols to enhance prognostic accuracy and patient management strategies. These studies collectively suggest the potential importance of thrombotic and inflammatory markers in risk stratification of patients experiencing COVID-19-related arrhythmias, but further work is required to establish the clinical utility of these biomarkers in different clinical contexts.
Conclusions
In conclusion, we provide evidence that specific inflammatory markers, including CRP, ferritin, leukocyte counts, PLR, and D-dimer levels are substantial prognostic factors in COVID-19 patients with AF. High levels of these markers in patients with the arrhythmic process indicate a higher inflammatory and thrombotic reaction to COVID-19, which may be the cause of the severity of the disease itself. Moreover, the decline in oxygen saturation in all of these patients suggests that arrhythmias may also adversely influence respiratory function. The striking increase in IL-6 in arrhythmogenic patients correlates with the trends seen in CT scores and length of hospitalization and is of clinical importance. These findings raise the possibility that early, rather than late anti-inflammatory treatments, such as the use of IL-6 antagonists, which have proven useful in severe COVID-19, may be helpful to patients with arrhythmia.
We advocate for close monitoring of these patients’ inflammatory status and suggest considering serial IL-6 monitoring as eventually part of the therapeutic strategy. Our results highlight the necessity of careful tracking inflammatory and coagulation pathways in patients with pre-existing arrhythmic milieu of COVID-19 to customize treatment approaches and potentially improve outcomes. These associations need to be further investigated to understand the mechanisms mediating them, and the potential validation of these markers should be made in larger, more diverse cohorts.
Acknowledgments
The authors express their profound gratitude to Chapidze Clinic for their dedicated efforts, and to all staff members who worked tirelessly during the challenging times of the COVID-19 pandemic. The exceptional commitment of healthcare professionals worldwide, particularly during such unprecedented circumstances, is deeply acknowledged and valued. This work is especially significant given the considerable demands placed on technical, financial, and intellectual resources. The authors extend their heartfelt appreciation to all involved, recognizing that, for a small country like Georgia, conducting high-level research of this caliber is both a commendable and invaluable achievement.
Financial Disclosure
This study was supported by Tbilisi State Medical University under the special program "Procedures for Encouraging Young Doctoral Students and Enhancing the Attractiveness of Doctoral Studies for Young Researchers”.
Conflict of Interest
The authors declare no conflict of interest. The founders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Informed Consent
All patients provided informed consent.
Author Contributions
Giorgi Tcholadze conceived and designed the study, conducted the research and collected all data, performed the analysis and interpretation of results, and drafted and finalized the manuscript. Ia Pantsulaia assisted in statistical analysis and interpretation of data, edited the manuscript and contributed to the preparation of figures, and provided critical feedback on the body text and overall study structure. Levan Ratiani assisted in patient recruitment and data collection, provided clinical insights and facilitated collaboration with study participants. Avtandil Jorbenadze facilitated access to patient samples and contributed to study logistics, provided practical assistance in patient management and organizational matters. Lela Kopaleishvili and Tamar Bolotashvili facilitated access to patient samples and contributed to study logistics, provided practical assistance in patient management and organizational matters. Tinatin Chikovani supported patient recruitment and helped in sample acquisition, provided logistical assistance and contributed to coordinating study activities.
Data Availability
The data presented in this study are available on reasonable request from the corresponding author.
| References | ▴Top |
- World Health Organization. "Weekly epidemiological update on COVID-19." https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---19-january-2023.
- Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811-818.
doi pubmed - Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):819-824.
doi pubmed - Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J Am Coll Cardiol. 2020;76(5):533-546.
doi pubmed pmc - Mohammad M, Emin M, Bhutta A, Gul EH, Voorhees E, Afzal MR. Cardiac arrhythmias associated with COVID-19 infection: state of the art review. Expert Rev Cardiovasc Ther. 2021;19(10):881-889.
doi pubmed - Bhatla A, Mayer MM, Adusumalli S, Hyman MC, Oh E, Tierney A, Moss J, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439-1444.
doi pubmed - Pannucci P, Jefferson SR, Hampshire J, Cooper SL, Hill SJ, Woolard J. COVID-19-Induced Myocarditis: Pathophysiological Roles of ACE2 and Toll-like Receptors. Int J Mol Sci. 2023;24(6):5374.
doi pubmed - Capecchi PL, Laghi-Pasini F, El-Sherif N, Qu Y, Boutjdir M, Lazzerini PE. Autoimmune and inflammatory K(+) channelopathies in cardiac arrhythmias: Clinical evidence and molecular mechanisms. Heart Rhythm. 2019;16(8):1273-1280.
doi pubmed - Rainer PP, Hao S, Vanhoutte D, Lee DI, Koitabashi N, Molkentin JD, Kass DA. Cardiomyocyte-specific transforming growth factor beta suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction. Circ Res. 2014;114(8):1246-1257.
doi pubmed pmc - Hanna A, Frangogiannis NG. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc Drugs Ther. 2020;34(6):849-863.
doi pubmed pmc - Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac Fibrosis: The Fibroblast Awakens. Circ Res. 2016;118(6):1021-1040.
doi pubmed - Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846-848.
doi pubmed - Fouda S, Hammond R, Donnelly PD, Coates ARM, Liu A. COVID-19 Pathophysiology: Inflammation to Cardiac Injury. Hearts. 2024;5(4):628-644.
doi - Tan C, Huang Y, Shi F, Tan K, Ma Q, Chen Y, Jiang X, et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. 2020;92(7):856-862.
doi pubmed pmc - Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147.
doi pubmed - Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
doi pubmed - Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown TS, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352-2371.
doi pubmed - Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-847.
doi pubmed - Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950-2973.
doi pubmed - Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040.
doi pubmed - Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, Luo M, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81(1):e6-e12.
doi pubmed - Qu R, Ling Y, Zhang YH, Wei LY, Chen X, Li XM, Liu XY, et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J Med Virol. 2020;92(9):1533-1541.
doi pubmed - Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017-1032.
doi pubmed - Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810.
doi pubmed - Zhang H, Dhalla NS. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int J Mol Sci. 2024;25(2):1082.
doi pubmed pmc - Saha SA, Russo AM, Chung MK, Deering TF, Lakkireddy D, Gopinathannair R. COVID-19 and Cardiac Arrhythmias: a Contemporary Review. Curr Treat Options Cardiovasc Med. 2022;24(6):87-107.
doi pubmed pmc - Kyala NJ, Mboya I, Shao E, Sakita F, Kilonzo KG, Shirima L, Sadiq A, et al. Neutrophil-to-lymphocyte ratio as a prognostic indicator in COVID-19: Evidence from a northern tanzanian cohort. PLoS One. 2025;20(1):e0300231.
doi pubmed pmc - Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, Zhang M, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18(1):206.
doi pubmed pmc - Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504.
doi pubmed pmc - Mihajlovic A, Ivanov D, Tapavicki B, Markovic M, Vukas D, Miljkovic A, Bajic D, et al. Prognostic Value of Routine Biomarkers in the Early Stage of COVID-19. Healthcare (Basel). 2023;11(15):2137.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.