| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 000, Number 000, April 2025, pages 000-000
Achieving Neuroprotection in the Setting of Early Extubation During Infant Cardiac Surgery: A Prospective, Randomized, and Blinded Study
Aymen N. Naguiba, b, d, Marc Bozycha, Kelly McNallyc, Mark Galantowiczb, Joseph Tobiasa
aDepartments of Anesthesiology and Pediatrics, Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH, USA
bThe Heart Center, Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH, USA
cDepartment of Pediatric Psychology and Neuropsychology, Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH, USA
dCorresponding Author: Aymen N. Naguib, The Heart Center, Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH 43205, USA
Manuscript submitted February 14, 2025, accepted April 10, 2025, published online April 22, 2025
Short title: Early Extubation After Pediatric Cardiac Surgery
doi: https://doi.org/10.14740/cr2029
| Abstract | ▴Top |
Background: This study aimed to investigate the impact of early extubation on stress response and neurodevelopmental outcomes after pediatric cardiac surgery utilizing cardiopulmonary bypass (CPB).
Methods: In this single-center prospective pilot study, we attempted to study the impact of using dexmedetomidine as an adjunct to facilitate early extubation after pediatric cardiac surgery requiring CPB during the first year of life. The study was conducted between May 2014 and January 2020. Perioperative data and stress hormone levels were collected at different points during the perioperative period. In addition, neurodevelopmental outcome measures including cognitive composite score, language (expressive and receptive) and motor (fine and gross) composites were evaluated at five time points including prior to surgery and up to 1 year after the procedure. Two-sample t-tests and Kruskal-Wallis tests were used to compare continuous parametric and non-parametric outcomes, respectively. Fisher’s exact or Chi-squared tests were used to compare categorical outcomes.
Results: A total of 30 subjects were included in the final cohort of patients. Of the 30 subjects, 14 patients were randomized to the dexmedetomidine group (dexmedetomidine plus fentanyl) (DEX group) and 16 patients were randomized to the no dexmedetomidine group (fentanyl only) (no DEX group). With few exceptions, both groups demonstrated appropriate blunting of the stress response. There was a significant increase in the ratio of the pro-inflammatory interleukin-10 (IL-10) to the anti-inflammatory interleukin-6 (IL-6) for the no DEX group at the end of the procedure when compared to the DEX group (10 ± 9 vs. 5 ± 4, P = 0.04). When looking at the Bayley cognitive composite score, the DEX group scored better than the no DEX group during the second visit (102 ± 11 vs. 88 ± 17, P = 0.023). By the fifth visit, the two groups scored similarly (94 ± 12 vs. 94 ± 12, P = 0.9 for the no DEX and DEX groups, respectively).
Conclusion: When looking at the neurodevelopmental outcome, our cohort had no significant changes in their Bayley scores from baseline with blunting of most stress markers. This study offers possible evidence of the safety of early extubation after pediatric cardiac surgery while maintaining the goal of neuroprotection.
Keywords: Fast tracking; Early extubation in pediatric cardiac surgery; Stress response; Neurodevelopmental outcome
| Introduction | ▴Top |
Early tracheal extubation after surgery for congenital heart disease (CHD) is a goal that has been analyzed and reviewed in the literature [1-4]. This practice has been shown to be safe, with the ultimate intent of decreasing morbidity and shortening intensive care unit (ICU) and total hospital lengths of stay. In addition, this strategy appears to be in line with the objective of neuroprotection, due to the expected decrease in exposure during the perioperative period to sedative medications, which are associated with potential adverse impacts on long-term neurocognitive outcomes. These concerns are most critical during the first 3 years of life, and particularly with exposures to gamma-amino butyric acid (GABA) agonists such as propofol, barbiturates, and isoflurane, as well as medications that decrease excitatory transmission through N-methyl-D-aspartic acid (NMDA) glutamate receptors, such as nitrous oxide and ketamine [5-7]. It has been hypothesized that these agents may trigger widespread neuronal apoptosis and eventually neurodegeneration during this vulnerable period of brain development.
Additionally, the stress response and associated inflammation have been shown to result in increased morbidity and mortality during the postoperative recovery period [8-10]. Due to their delicate metabolic balance, neonates and infants undergoing cardiac surgery are at greatest risk for experiencing complications and poor outcomes secondary to the surgical stress response and inflammation. While the stress response plays a major role in initiating the catabolic state that follows surgery, cytokine release and the production of pro-inflammatory mediators contribute to end-organ dysfunction [11]. The cytokines are produced mainly in the lungs and myocardium, and then released into the circulation [11]. These include pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), and interleukin-8 (IL-8). There are also compensatory anti-inflammatory cytokines, such as interleukin-10 (IL-10) and interleukin-1 receptor antagonist (IL-1ra).
The pro-inflammatory cytokine response in children undergoing cardiac surgery with cardiopulmonary bypass (CPB) is characterized by wide variation, when compared to a generally well-defined response in adults [12]. This is in contrast to the anti-inflammatory response that shows a more clear release pattern in children. The balance between the pro- and anti-inflammatory cytokines may help to predict outcomes for children undergoing cardiac surgery with CPB [13, 14]. In addition, increased plasma levels of biomarkers such as S-100 protein beta chain (S100B) and neuron-specific enolase (NSE) have been linked to neurological injury and poor neurological outcomes following ischemic brain injury [15, 16]. Other studies have shown that increased S100B and NSE levels during and after cardiac surgery in adults were correlated with worse neurological outcomes. The S100B is a calcium binding protein that is normally found in the intra- and extracellular brain tissue. Its presence in the plasma indicates disruption of the blood-brain barrier and brain injury, and it is eliminated by the kidney with a half-life of 1 - 2 h. On the other hand, NSE is an intracytoplasmic glycolytic enzyme that is found in neurons and neuroendocrine cells, and detection of NSE in the plasma indicates high death rate of these cells.
Recent in vitro and in vivo animal studies have shown the possible neuroprotective effect of dexmedetomidine, a selective α2-adrenergic agonist [17]. Degos et al showed that the use of dexmedetomidine in mouse models provided modulation of brain-derived neurotrophic factor expression [18]. This modulation resulted in significant neuroprotective effects in vivo and in vitro. For these reasons, a balance between early tracheal extubation in order to minimize exposure to these anesthetic medications and abolishing (or at least blunting) the stress response appears to be of paramount importance. In addition, identifying a specific anesthetic regimen that will provide some neuroprotection while offering some blunting of the stress response may improve both immediate perioperative and long-term neurodevelopmental outcomes in children undergoing cardiac surgery with CPB under 1 year of age.
In a previous pilot study at our institution, we demonstrated that the use of fentanyl at a dose greater than 10 µg/kg, alone or with the addition of dexmedetomidine, was associated with a better blunting of the stress response [18]. In a follow-up neurodevelopmental study, patients with greater blunting of the stress response experienced better neurodevelopmental outcomes, although the results did not reach statistical significance in some cases, likely due to the small sample size [19]. In this current prospective, randomized, and blinded study, we hypothesized that the addition of dexmedetomidine to the anesthetic would result in better blunting of the stress response perioperatively, and may offer more neuroprotection to infants undergoing cardiac surgery utilizing CPB.
| Materials and Methods | ▴Top |
The Institutional Review Board (IRB) at Nationwide Children’s Hospital approved this study (IRB# 13-00088). An Investigational New Drug (IND) application was obtained (IND# 101911), and the study was registered at ClinicalTrials.gov (ID NCT 02492269). The study was conducted between May 2014 and January 2020. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Inclusion criteria comprised patients between 1 month and 1 year of age undergoing repair of atrial septal defect (ASD), ventricular septal defect (VSD), atrioventricular septal defect (AVSD), tetralogy of Fallot (TOF), or biventricular repair with left-to-right-shunting physiology. We excluded any patient with the above diagnoses who received previous anesthetic or had any other comorbidity or genetic disorder. Potential subjects were recruited from the cardiology and preadmission testing (PAT) clinics. Patients were randomly assigned by the operating room (OR) pharmacy to either receive fentanyl at 15 µg/kg in addition to dexmedetomidine at a loading dose of 1 µg/kg followed by an infusion of 0.5 µg/kg/h until the conclusion of CPB (DEX group), or only fentanyl at 15 µg/kg (no DEX group). The dose of dexmedetomidine selected is the standard dose at our institution and was used in our previous studies [18]. All patients were maintained on isoflurane combined with the study drugs. Isoflurane was titrated during the procedure at the discretion of the attending cardiac anesthesiologist.
Study drugs protocol
At the start of the case, the attending anesthesiologist received two syringes containing the study drugs. The first syringe was labeled, “study drug - fentanyl.” Half of the drug volume was administered at the induction of anesthesia after obtaining intravenous (IV) access, and the second half was administered prior to skin incision. The second syringe was labeled, “study drug - dexmedetomidine/placebo at a concentration of 4 µg/mL,” and contained either dexmedetomidine (4 µg/mL) or normal saline. The dexmedetomidine/placebo was administered after obtaining IV access at a loading dose of 1 µg/kg over 10 min, followed by an infusion at a rate of 0.5 µg/kg/h, being programmed into the infusion pump as though it were dexmedetomidine (4 µg/mL) regardless. The dexmedetomidine/placebo infusion was discontinued after separation from CPB.
Blood collection and handling
Blood samples were drawn from an existing arterial line.
Arterial blood gases, glucose, and lactate levels were recorded at each of the phlebotomy time points.
Blood of 3 mL was collected at five time points: after the induction of anesthesia (baseline), after sternotomy, after initiation of CPB, at the conclusion of surgery (separation from CPB and administration of protamine, and prior to skin closure), and at 24 h postoperatively. The S100B and NSE levels were tested at baseline, end of surgery, and 24 h after surgery.
Blood samples were analyzed for relative amounts of cortisol, epinephrine, norepinephrine, adrenocorticotropic hormone (ACTH), nitrated albumin, and the cytokines TNF-α, IL-8, IL-6, and IL-10.
Zero balance ultrafiltrate (ZBUF) and modified ultrafiltrate (MUF) fluids were collected at the conclusion of surgery and analyzed for the cytokines TNF-α, IL-8, IL-6, and IL-10. Samples of ZBUF and MUF fluids were collected after separation from CPB and at the conclusion of MUF, respectively. Blood samples were collected in tubes with ethylene diamine tetra acetic acid (EDTA) preservative and centrifuged at 1,500 × g at 4 °C for 10 min. The plasma was then separated into aliquots and stored at -80 °C until the time of analysis, at which point it was thawed on ice and centrifuged at 14,000 × g for 1 min prior to testing.
For hormonal assays, ACTH and cortisol were measured by enzyme-linked immunosorbent assay (ELISA) (Cal Biotech, Spring Valley, CA). Epinephrine and norepinephrine were also evaluated by ELISA (2-CAT; Rocky Mountain Scientific, Centennial, CO). All assays were performed according to the manufacturer’s instructions.
For cytokine assays, cytokine levels in plasma and MUF samples were measured at The Research Institute at Nationwide Children’s Hospital using the Meso Scale cytokine multiplex ELISA format (Meso Scale Diagnostics, Rockville, MD). Measured cytokines included: interferon gamma (IFN-γ), IL-1β, IL-10, IL-12 p70, IL-6, IL-8, and TNF-α. Manufacturer-provided standards and controls were included in each run to generate calibration curves.
Plasma S100B levels were measured by colorimetric ELISA analysis using a commercially available kit (Bio Vendor R&D, Inc., Candler, NC) with included calibration controls.
Plasma NSE levels were measured using a Quantikine ELISA kit (R&D Systems, Inc., Minneapolis, MN) with included calibration controls.
Tracheal extubation criteria
Provided that there were no preoperative contraindications for early tracheal extubation, the trachea was extubated at the conclusion of the surgical procedure according to set criteria at our institution [1, 2]. After separation from CPB, reversal of heparin with protamine, and achievement of hemostasis (assuming good biventricular function on post-CPB transesophageal echocardiogram and hemodynamic stability), the chest was closed. If the patient continued to maintain stable hemodynamic performance, a trial of spontaneous ventilation was allowed, following reversal of residual neuromuscular blockade. If the patient remained hemodynamically stable with acceptable respiratory rate, tidal volume, and expected arterial oxygen saturation at the conclusion of the procedure, the trachea was extubated.
Intraoperative data collection
Collected data included: age, weight, sex, diagnosis, induction time (from the start of the induction of anesthesia until first blood gas), bispectral index (BIS) value, cerebral saturation data, baseline hemoglobin, baseline platelet count, acute normovolemic hemodilution (ANH) volume prior to CPB, CPB circuit prime constituents, red blood cell (RBC) administration, additional blood products (platelet, cryoprecipitate, and/or plasma) and total volume of each product given, ZBUF and MUF volumes, CPB time, aortic cross clamp time, and the time of tracheal extubation (if applicable).
Postoperative cardiothoracic intensive care unit (CTICU) data collection
Collected data included: length of positive pressure ventilation support, total chest tube output during the first 24 h postoperatively, postoperative platelet count, postoperative absolute neutrophil count, and postoperative coagulation profile including prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR), and fibrinogen level. Data also included inotropic score upon arrival and at 24 h postoperatively. The inotropic score was calculated as follows: dopamine (µg/kg/min) + milrinone (µg/kg/min) × 10 + epinephrine (µg/kg/min) × 100 [20].
Other data collected included: total urine output during the first 24 h postoperatively, blood urea nitrogen (BUN) and creatinine upon arrival and at 24 h postoperatively, and the use of nurse-controlled analgesia (NCA), including starting opioid, the need to change the medication used, and the total dose of opioids for the first 24 h.
Other variables included: cardiac arrest requiring resuscitation, ventricular or atrial arrhythmia causing hemodynamic disturbances which required treatment, nosocomial infection during the hospitalization, disseminated intravascular coagulation (DIC), clinical evidence of seizures, the need for re-intubation, length of ICU stay, the need for transfusing blood or blood products (and total volume given), length of hospital stay, and postoperative mortality.
Neurodevelopmental testing
The primary neurodevelopmental outcome measure was the cognitive composite from The Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) [21-23]. Secondary outcomes included the language (expressive and receptive) and motor (fine and gross) composites. The Bayley-III was administered by trained technicians under the supervision of a licensed psychologist. The Bayley-III was administered at five time points throughout the study (preoperatively, postoperatively before discharge from hospital, 1 - 3 months postoperatively, 6 months postoperatively, and 1 year postoperatively). The motor subtests were not administered at the visit prior to discharge from the hospital due to limitations on standardized administration of these items immediately following surgery.
Parent-reported adaptive functioning was also assessed using the Adaptive Behavior Assessment System, Second Edition (ABAS-II) Parent/Primary Caregiver Form (Ages 0-5) [24]. This is a parent-reported questionnaire that assesses adaptive functioning in the domains of conceptual, social, and practical skills. The General Ability Composite (GAC) was used as the primary outcome from the ABAS-II, and was administered to one parent at four time points (preoperatively, 1 to 3 months postoperatively, 6 months postoperatively, and 1 year postoperatively). The ABAS-II was not administered at the second time point (i.e., prior to discharge from the hospital) due to this visit following so closely after the baseline visit.
Subjects received $10 for the baseline visit and $25 for each subsequent study visit: prior to discharge from the hospital, and 1 to 3 months, 6 months, and 1 year postoperatively.
Statistical methods
Continuous data were presented as means with standard deviations or medians with interquartile ranges. Categorical variables were presented as frequencies and percentages. Two-sample t-tests and Kruskal-Wallis tests were used to compare continuous parametric and non-parametric outcomes, respectively. Fisher’s exact or Chi-squared tests were used to compare categorical outcomes. Repeated measure analysis of variance (ANOVA) was used to compare the neurodevelopmental scores and stress marker values between the dexmedetomidine and saline placebo groups over time. Multiple comparisons were corrected by controlling the false discovery rate using a two-stage step-up method. All statistical analyses were performed using Python (Python Software Foundation, Wilmington, DE) and Graph Pad Prism 9.0.0 (GraphPad Software, San Diego, CA).
| Results | ▴Top |
With the start of the COVID pandemic, our process of in-person PAT was halted, which prevented recruitment of the desired 25 patients in each group, and so we ended with a final study cohort of only 30 subjects. Of the 30, 14 patients were randomized to the DEX group and 16 to the no DEX group (Fig. 1). There were no differences in age or weight between the two groups (Table 1). The no DEX group included six female and 10 male children, while the DEX group included four female and 10 male. The cohort included 15 patients (50%) with the diagnosis of TOF (nine in the no DEX group and six in the DEX group). There were 12 patients (43%) who had the diagnosis of VSD (five in the no DEX group and seven in the DEX group). Three patients (7%) had the diagnosis of AVSD (two patients in the no DEX group and one patient in the DEX group) (Table 1). No statistical differences were found in patient demographics between the DEX and no DEX groups. It is worth mentioning that three patients in the no DEX group and one patient in the DEX group were prescribed beta blockers prior to the procedure.
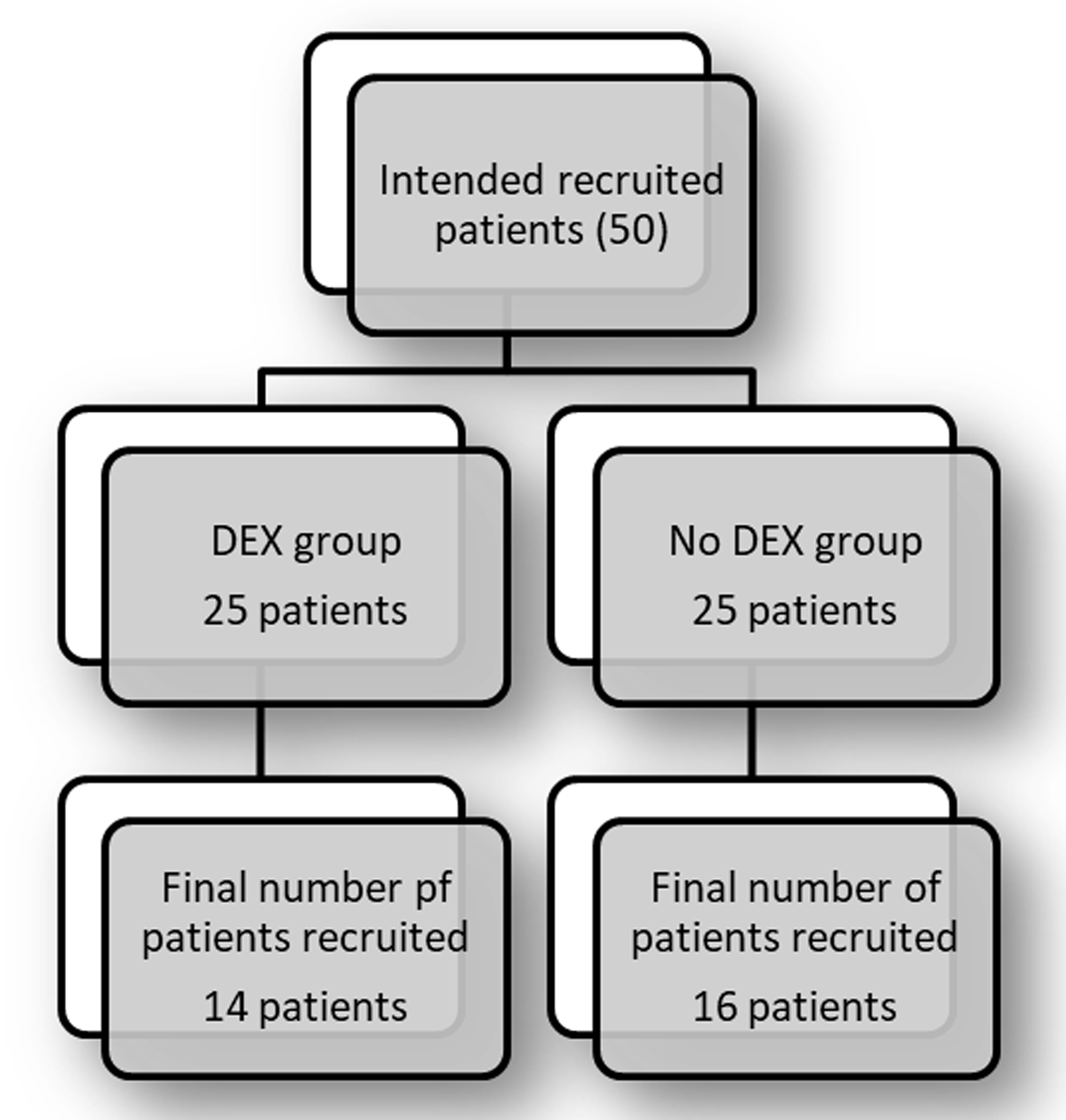 Click for large image | Figure 1. Flow chart. |
 Click to view | Table 1. Patient Demographics |
OR procedural information is displayed in Table 2. There were no significant differences between the two groups in terms of the number of subjects undergoing ANH, the volume of ANH, total bypass time, and total cross clamp time. Additionally, no differences in the washed RBC, MUF, and ZBUF volumes were detected. The only significant difference was in blood pressure readings after sternotomy, where the DEX group had significantly lower systolic, diastolic, and mean blood pressures compared to the no DEX group. Mean blood pressure was 45 ± 10 mm Hg in the DEX group and 56 ± 10 mm Hg in the no DEX group (P = 0.004). There were no differences between the two groups regarding lactate or glucose measurements at the different blood drawing points.
 Click to view | Table 2. OR Information |
There were no statistically significant differences between the two groups in plasma levels of TNF-α, IL-6, IL-8, and IL-10 (Fig. 2). There was a significant increase in the ratio of the pro-inflammatory IL-10 to the anti-inflammatory IL-6 for the no DEX group at the end of the procedure, when compared to the DEX group (10 ± 9 vs. 5 ± 4, P = 0.04; Fig. 2). In addition, while there were no significant differences between the two groups for the intraoperative levels of the stress hormones ACTH, cortisol, epinephrine, and norepinephrine, the cortisol level 24 h after surgery was significantly lower in the no DEX group, as compared to the DEX group (182 ± 225 vs. 531 ± 489 ng/mL, P = 0.025). Also, cortisol index delta (Δ), which represents the change from baseline as measured 24 h after surgery, was significantly lower in the no DEX group (-224 ± 275 vs. 200 ± 489 ng/mL, P = 0.009; Fig. 2). There was no significant difference between the two groups in relation to the S100B and NSE levels throughout (Fig. 3). At the end of surgery, NSE had significantly increased for both groups, compared to baseline (9 ± 4 ng/mL, P < 0.0001 for the no DEX group and 10 ± 12 ng/mL, P = 0.007 for the DEX group). The NSE levels continued to be significantly elevated from baseline in both groups at 24 h post-procedure (9 ± 4 ng/mL, P = 0.00002 for the no DEX group and 10 ± 12 ng/mL, P = 0.0006 for the DEX group). The S100B levels had a similar response. Post-procedure, the no DEX group had an increase of 90 ± 132 pg/mL, P = 0.05 from baseline and the DEX group levels were 108 ± 90 pg/mL, P = 0.004 higher than baseline. At 24 h, the S100B levels remained higher than baseline, although this did not reach statistical significance.
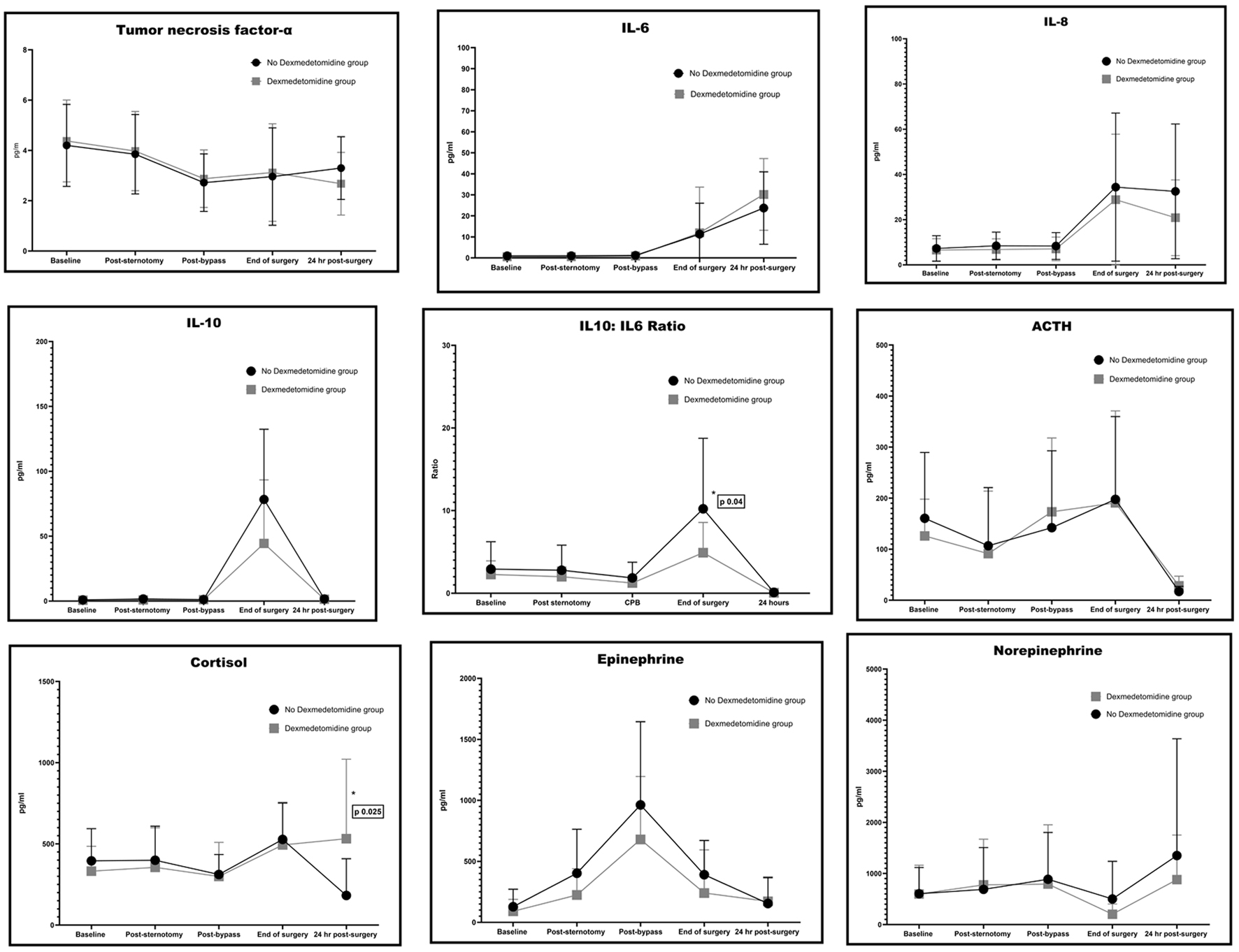 Click for large image | Figure 2. Cytokines and hormones assays. |
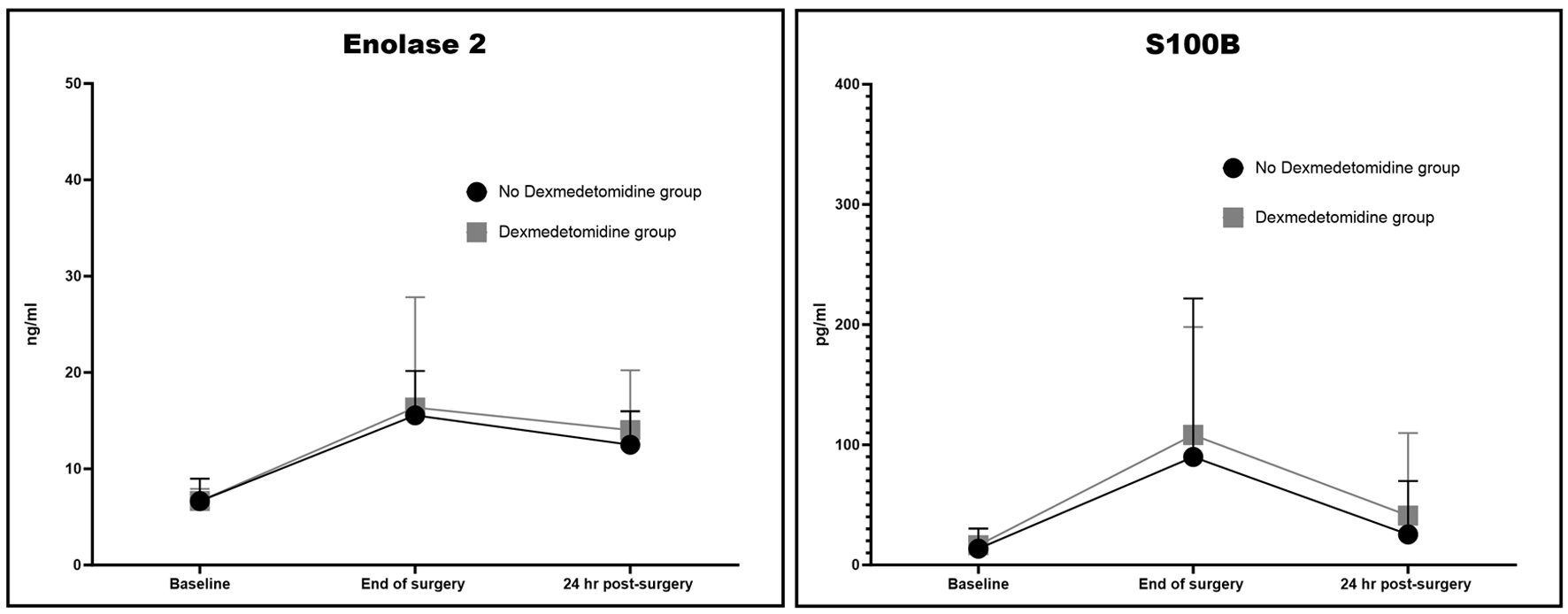 Click for large image | Figure 3. Neuron-specific enolase (NSE) and S-100 protein beta chain (S100B). |
Information pertaining to the CTICU is displayed in Table 3. There was no significant difference between the two groups regarding 24-h chest tube output (18 ± 7 vs. 18 ± 8 mL/kg, P = 0.8 for the no DEX and DEX groups, respectively). Similarly, there was no difference between the two groups regarding the 24-h urine output and total opioid dose. For example, the total fentanyl dose for the first 24 h was 17 ± 12 vs. 18 ± 9 µg/kg, P = 0.7 for the no DEX and DEX groups, respectively. There were also no differences in the CTICU lengths of stay (2 ± 2 vs. 2 ± 1 days, P = 0.7) or hospital lengths of stay (6 ± 2 vs. 6 ± 3 days, P = 0.5) for the no DEX and DEX groups, respectively. Additionally, no differences were detected in inotropic score, BUN, or creatinine on arrival or 24 h postoperatively (Table 3). Finally, there were no differences in fibrinogen, lactate, or glucose measurements between the two groups. The no DEX group included one patient who had to be reintubated and one patient who experienced a cardiac arrest on postoperative day 2. Two patients in the no DEX group and one patient in the DEX group developed arrhythmias. No patients experienced nosocomial infection, DIC, or seizures. Four patients in the no DEX group and three patients in the DEX group received additional blood or blood products in the CTICU. No statistical differences were detected in these clinical outcomes.
 Click to view | Table 3. CTICU Information |
Neurodevelopmental outcomes are shown in Figure 4. For cognitive functioning (Bayley cognitive composite), there was not a significant group difference at baseline. However, immediately post-surgery, the no DEX group showed post-surgical declines in cognitive performance, resulting in group differences at visit 2 (102 ± 11 vs. 88 ± 17, P = 0.023). Cognitive performance rebounded to pre-surgical level by the 1 - 3 months post-surgery time point and there were no group differences between DEX and no DEX groups for the remaining time points. For language functioning, at baseline, the no DEX group performed lower than the DEX group and this difference was maintained immediately post-surgery (stats). However, by 1 - 3 months post-surgery, no further group differences were seen and this was maintained for subsequent visits (stats). For motor functioning, there were no significant group differences across any time points. Of note, acute post-surgical motor functioning could not be assessed due to limitations of testing in the inpatient post-acute surgery setting.
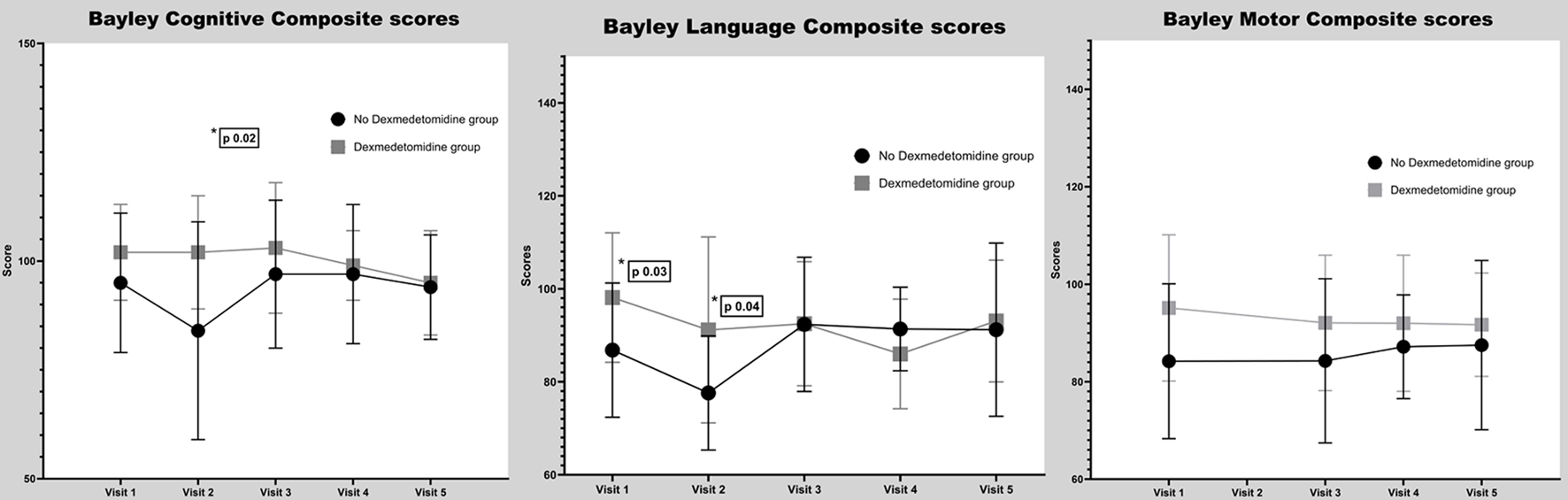 Click for large image | Figure 4. Bayley scores. |
For parent-reported adaptive living skills, there were no significant group differences across time points (Fig. 5). Parent ratings of adaptive behavior were not gathered in the acute post-surgery time frame.
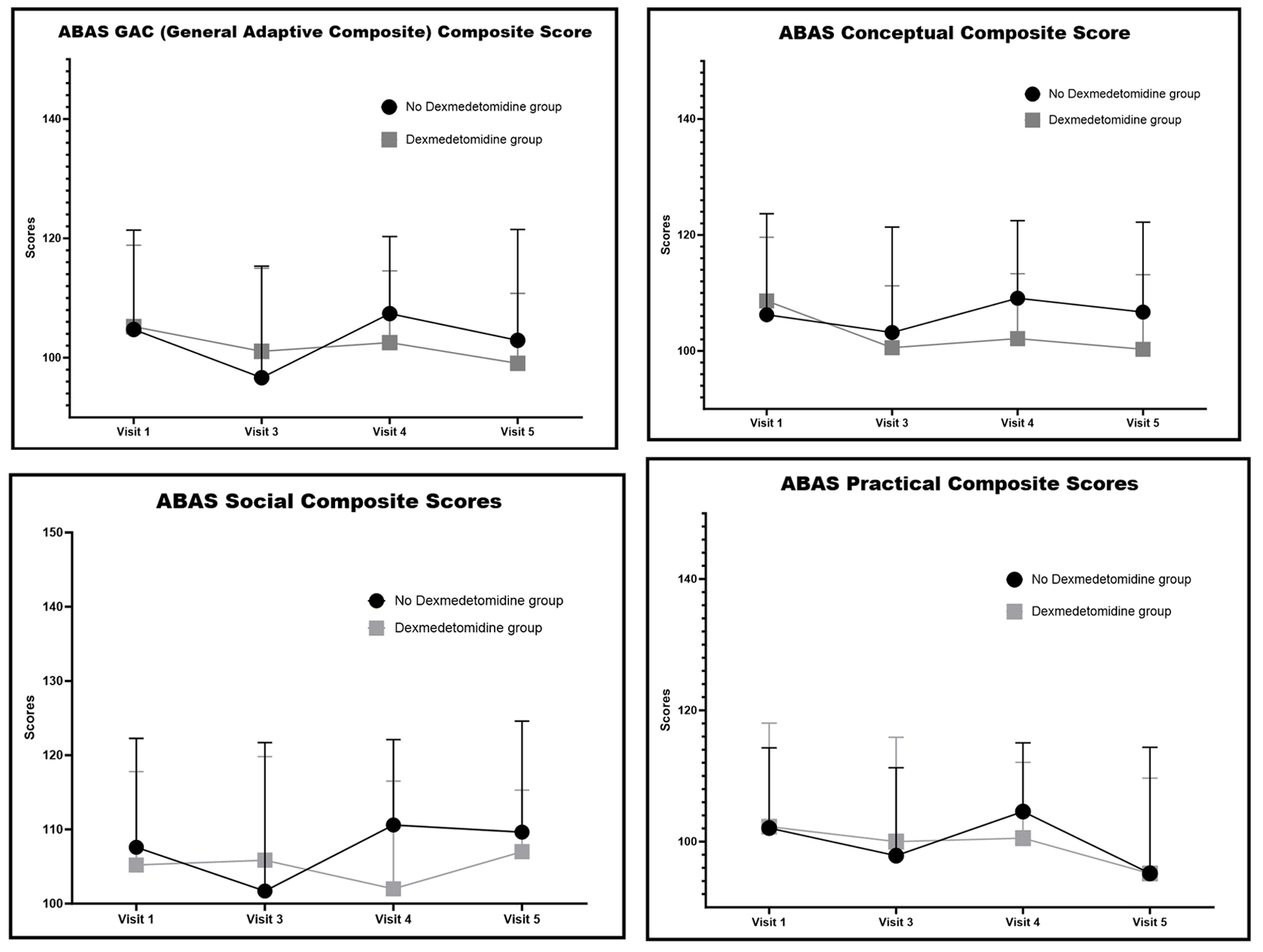 Click for large image | Figure 5. Adaptive Behavior Assessment System, Second Edition (ABAS-II) scores. |
| Discussion | ▴Top |
In a continuation of our efforts to achieve optimal patient outcomes, we have tried to balance our existing practice of early tracheal extubation after surgery for CHD with the ultimate goals of additionally improving the immediate perioperative course and long-term neurodevelopmental outcomes. While Anand and colleagues [8] laid the foundation for the nature of the stress response and described their attempt to ensure proper blunting of this response during cardiac surgery, other data emerged that linked specific anesthetic medications to a possible impact on the long-term neurodevelopmental outcome of these children, especially during the first 3 years of life [5-7]. Therefore, it is imperative to balance blunting of the stress response with the ultimate goal of ensuring positive long-term neurodevelopmental outcomes.
In a previous study at our institution, we reported that the addition of dexmedetomidine to a lower dose fentanyl blunted the stress response in a fashion comparable to a high-dose fentanyl technique [18]. In the current study, we have attempted to further investigate these outcomes by evaluating the impact of adding dexmedetomidine to a higher-dose fentanyl technique on both the stress response and long-term neurodevelopmental outcomes. With few exceptions, both groups demonstrated appropriate blunting of the stress response. The stress hormones TNF-α, cortisol, and ACTH stayed at or below baseline levels in both groups.
Cortisol level at 24 h post-procedure was lower in the no DEX group compared to baseline and to the DEX group. Some studies point to the correlation of high cortisol level upon ICU admission and the complexity of the ICU stay in children [24]. Other studies shows that cortisol level usually peaks at 24 h post-procedure and that level recovers slowly in patients with high cardiac risk compared to their counterparts [25]. In addition, additional studies indicate that low cortisol level could be the result of adrenal insufficiency that is associated with low cardiac and urine outputs [26, 27]. Neither of our two groups demonstrated hemodynamic instability, as evident by low inotropic scores, good urine output, and short CTICU and hospital lengths of stay.
When it comes to the pro-inflammatory cytokines, while both IL-6 and IL-8 levels were significantly increased from baseline in both groups at the end of the procedure and 24 h after, there was no significant difference between the two groups. The only difference was the ratio between the anti-inflammatory cytokine IL-10 and the pro-inflammatory cytokine IL-6 at the end of surgery, where the DEX group had a lower IL-10:IL-6 ratio compared to the no DEX group. Again, while a lower ratio has been shown to be associated with a more severe systemic inflammatory response, increased incidence of postoperative arrhythmias, and longer CTICU and hospital lengths of stay [14], our two groups demonstrated shorter CTICU and hospital lengths of stay. Our two patients who developed postoperative rhythm issues had junctional ectopic tachycardia and complete heart block, which were known possible complications of their surgical repairs and cardiac lesions. When evaluating epinephrine and norepinephrine levels, as expected, dexmedetomidine use resulted in lower levels at different time points, although these findings did not reach statistical significance.
As expected, post-procedure levels of S100B rose from baseline in both groups, and then started to drop at 24 h post-procedure. The rise in S100B may be associated with delirium, cognitive impairment, and prolonged hospital stay [28]. Again, this was not demonstrated in our cohort of patients, who all had very short hospital stays and no reported post-procedure delirium. It is important to note that S100B level may not be a very specific brain injury marker, and its rise may be due to factors like traumatized mediastinal fat and skeletal muscle from the surgical wound [24]. The use of inhalational anesthetic agents has also been found to interfere with S100B level [25].
When looking at neurodevelopmental outcomes, our cohort had no significant changes in their Bayley scores from baseline. Even though the no DEX group demonstrated a significant drop in their Bayley cognitive and language composite scores in the immediate postoperative visit, these scores normalized by the 3-month visit. When compared to the general population, our cohort scored slightly lower on these metrics.
Limitations
One clear weakness of our study is the small number of patients included in the final cohort, due to our inability to secure the intended total number of participants. The start of the COVID-19 pandemic and associated change in our PAT process played a major role in preventing us from reaching this goal. While the small number of patients included in the final cohort prohibits us from drawing strong conclusions, our results are in line with and support other studies that found variation in the hormonal and cytokine responses in pediatric cardiac surgery, when compared to a more predictable response in the adult population. In addition, the lack of differences in the neurodevelopmental outcomes between the two groups and between the entire cohort and the general population is supported by other studies, which have demonstrated a greater impact from preoperative factors, like prematurity, socioeconomic status, and type of congenital cardiac lesions on the neurodevelopmental outcome than from perioperative factors [29-31].
Conclusions
While our cohort of patients was small to draw a definitive conclusion about the impact of dexmedetomidine on neurodevelopmental outcome, this study adds another possible step in understanding early extubation after pediatric cardiac surgery. This can be achieved without neglecting the stress response and the ultimate neurodevelopmental outcomes for this patient population.
Acknowledgments
The authors acknowledge Julie Rice-Weimer, BSN, RN, CCRC our lead research coordinator and the research team at the Department of Anesthesia and Pain Medicine for their work in recruiting patients and collecting the data. The authors would like to also acknowledge the Biobehavioral Outcomes Core Group at Nationwide Children’s Hospital for their help in performing and collecting data for the neurodevelopmental testing.
Financial Disclosure
Funding was obtained via a grant from the Heart Center Translational Research Fund at Nationwide Children’s Hospital (Grant#NCHAWD00008230).
Conflict of Interest
The authors declare that there is no conflict of interest.
Informed Consent
Written informed consent was also obtained from a parent or a legal guardian.
Author Contributions
Dr. Aymen Naguib was responsible for study design, data collection and analysis, and writing of the manuscript. Dr. Marc Bozych helped with the write up and editing. Dr Kelly McNally was involved with the neurodevelopmental testing, interpretation, and writing of the manuscript. Dr. Mark Galantowicz helped with write up and editing. Dr. Joseph Tobias helped with the write up and editing of the manuscript.
Data Availability
The authors declare that the data supporting the findings in this article are available within the article.
Abbreviations
ACTH: adrenocorticotropic hormone; ANH: acute normovolemic hemodilution; CHD: congenital heart disease; CPB: cardiopulmonary bypass; EDTA: ethylene diamine tetra acetic acid; GABA: gamma-amino butyric acid; ICU: intensive care unit; IL-1: interleukin-1; IL-6: interleukin-6; IL-8: interleukin-8; IL-10: interleukin-10; IL-1ra: interleukin-1 receptor antagonist; MUF: modified ultrafiltrate; NMDA: N-methyl-D-aspartic acid; NSE: neuron-specific enolase; S100B: S-100 protein beta chain; TNF-α: tumor necrosis factor alpha; ZBUF: zero balance ultrafiltrate
| References | ▴Top |
- Winch PD, Nicholson L, Isaacs J, Spanos S, Olshove V, Naguib A. Predictors of successful early extubation following congenital cardiac surgery in neonates and infants. Heart Lung Circ. 2009;18(4):271-276.
doi pubmed - Winch PD, Staudt AM, Sebastian R, Corridore M, Tumin D, Simsic J, Galantowicz M, et al. Learning from experience: improving early tracheal extubation success after congenital cardiac surgery. Pediatr Crit Care Med. 2016;17(7):630-637.
doi pubmed - Faraoni D, Ng WCK. Pro: early extubation after pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 2020;34(9):2539-2541.
doi pubmed - Iguidbashian JP, Chang PH, Iguidbashian J, Lines J, Maxwell BG. Enhanced recovery and early extubation after pediatric cardiac surgery using single-dose intravenous methadone. Ann Card Anaesth. 2020;23(1):70-74.
doi pubmed - Jevtovic-Todorovic V, Benshoff N, Olney JW. Ketamine potentiates cerebrocortical damage induced by the common anaesthetic agent nitrous oxide in adult rats. Br J Pharmacol. 2000;130(7):1692-1698.
doi pubmed - Jevtovic-Todorovic V. General anesthetics and the developing brain: friends or foes? J Neurosurg Anesthesiol. 2005;17(4):204-206.
doi pubmed - Jevtovic-Todorovic V, Carter LB. The anesthetics nitrous oxide and ketamine are more neurotoxic to old than to young rat brain. Neurobiol Aging. 2005;26(6):947-956.
doi pubmed - Anand KJ, Hickey PR. Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery. N Engl J Med. 1992;326(1):1-9.
doi pubmed - Hirai S, Hamanaka Y, Mitsui N, Isaka M, Sutoh M. [Prospective study of systemic inflammatory response syndrome after cardiac surgery as a effective indicator]. Kyobu Geka. 2004;57(6):455-458.
pubmed - Raja SG, Dreyfus GD. Modulation of systemic inflammatory response after cardiac surgery. Asian Cardiovasc Thorac Ann. 2005;13(4):382-395.
doi pubmed - Cusack B, Buggy DJ. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020;20(9):321-328.
doi pubmed - Bronicki RA, Hall M. Cardiopulmonary bypass-induced inflammatory response: pathophysiology and treatment. Pediatr Crit Care Med. 2016;17(8 Suppl 1):S272-278.
doi pubmed - Kim JA, Ahn HJ, Yang M, Lee SH, Jeong H, Seong BG. Intraoperative use of dexmedetomidine for the prevention of emergence agitation and postoperative delirium in thoracic surgery: a randomized-controlled trial. Can J Anaesth. 2019;66(4):371-379.
doi pubmed - Weis F, Beiras-Fernandez A, Schelling G, Briegel J, Lang P, Hauer D, Kreth S, et al. Stress doses of hydrocortisone in high-risk patients undergoing cardiac surgery: effects on interleukin-6 to interleukin-10 ratio and early outcome. Crit Care Med. 2009;37(5):1685-1690.
doi pubmed - Bar-Yosef O, Greidinger D, Iskilova M, Hemi R, Tirosh T, Vardi A. Neurological deficit is predicted by S100B in children after cardiac surgery. Clin Chim Acta. 2018;481:56-60.
doi pubmed - Topjian AA, Lin R, Morris MC, Ichord R, Drott H, Bayer CR, Helfaer MA, et al. Neuron-specific enolase and S-100B are associated with neurologic outcome after pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10(4):479-490.
doi pubmed - Unchiti K, Leurcharusmee P, Samerchua A, Pipanmekaporn T, Chattipakorn N, Chattipakorn SC. The potential role of dexmedetomidine on neuroprotection and its possible mechanisms: Evidence from in vitro and in vivo studies. Eur J Neurosci. 2021;54(9):7006-7047.
doi pubmed - Naguib AN, Tobias JD, Hall MW, Cismowski MJ, Miao Y, Barry N, Preston T, et al. The role of different anesthetic techniques in altering the stress response during cardiac surgery in children: a prospective, double-blinded, and randomized study. Pediatr Crit Care Med. 2013;14(5):481-490.
doi pubmed - Naguib AN, Winch PD, Tobias JD, Yeates KO, Miao Y, Galantowicz M, Hoffman TM. Neurodevelopmental outcome after cardiac surgery utilizing cardiopulmonary bypass in children. Saudi J Anaesth. 2015;9(1):12-18.
doi pubmed - Wernovsky G, Kuijpers M, Van Rossem MC, Marino BS, Ravishankar C, Dominguez T, Godinez RI, et al. Postoperative course in the cardiac intensive care unit following the first stage of Norwood reconstruction. Cardiol Young. 2007;17(6):652-665.
doi pubmed - Ballot DE, Ramdin T, Rakotsoane D, Agaba F, Davies VA, Chirwa T, Cooper PA. Use of the Bayley Scales of Infant and Toddler Development, third edition, to assess developmental outcome in infants and young children in an urban setting in South Africa. Int Sch Res Notices. 2017;2017:1631760.
doi pubmed - Del Rosario C, Slevin M, Molloy EJ, Quigley J, Nixon E. How to use the Bayley Scales of Infant and Toddler Development. Arch Dis Child Educ Pract Ed. 2021;106(2):108-112.
doi pubmed - Doh JH, Kim SA, Oh K, Kim Y, Park N, Park S, Heo NH. The predictive value of language scales: bayley scales of infant and toddler development third edition in correlation with Korean sequenced language scale for infant. Ann Rehabil Med. 2020;44(5):378-385.
doi pubmed - Plumpton KR, Anderson BJ, Beca J. Thyroid hormone and cortisol concentrations after congenital heart surgery in infants younger than 3 months of age. Intensive Care Med. 2010;36(2):321-328.
doi pubmed - Al-Sofyani KA, Uddin MS, Qulisy EA, Al-Radi OO. Patterns and determinants of change in cortisol levels and thyroid function as a function of cardiac risk in children undergoing cardiac surgery. Int J Pediatr. 2022;2022:6730666.
doi pubmed - Saiki H, Kuwata S, Kurishima C, Iwamoto Y, Ishido H, Masutani S, Senzaki H. Aldosterone-cortisol imbalance immediately after fontan operation with implications for abnormal fluid homeostasis. Am J Cardiol. 2014;114(10):1578-1583.
doi pubmed - Gajarski RJ, Stefanelli CB, Graziano JN, Kaciroti N, Charpie JR, Vazquez D. Adrenocortical response in infants undergoing cardiac surgery with cardiopulmonary bypass and circulatory arrest. Pediatr Crit Care Med. 2010;11(1):44-51.
doi pubmed - Lapergola G, Graziosi A, D'Adamo E, Brindisino P, Ferrari M, Romanelli A, Strozzi M, et al. S100B in cardiac surgery brain monitoring: friend or foe? Clin Chem Lab Med. 2022;60(3):317-331.
doi pubmed - Goff DA, Luan X, Gerdes M, Bernbaum J, D'Agostino JA, Rychik J, Wernovsky G, et al. Younger gestational age is associated with worse neurodevelopmental outcomes after cardiac surgery in infancy. J Thorac Cardiovasc Surg. 2012;143(3):535-542.
doi pubmed - Gaynor JW, Gerdes M, Nord AS, Bernbaum J, Zackai E, Wernovsky G, Clancy RR, et al. Is cardiac diagnosis a predictor of neurodevelopmental outcome after cardiac surgery in infancy? J Thorac Cardiovasc Surg. 2010;140(6):1230-1237.
doi pubmed - Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J, Gerdes M, Zackai E, Nord AS, et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2007;133(5):1344-1353.e3.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.