| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Review
Volume 16, Number 1, February 2025, pages 1-14
Beyond the Beat: A Multifaceted Review of Atrial Fibrillation in Sepsis: Risk Factors, Management Strategies, and Economic Impact
Wing Lam Hoa, i, Muhammad Umaisb, Meena Baic, Ngoc Bao Dangd, Kajal Kumarie, Sara Izharf, Rabia Asrarg, Toleen Haddadh, Muhammad Ali Muzammilg
aSt George’s University School of Medicine, West Indies, Grenada
bKing Edward Medical University, Lahore, Pakistan
cPeoples University of Medical and Health Sciences for Women Nawabshah, Sindh, Pakistan
dCollege of Health Sciences, VinUniversity, Hanoi, Vietnam
eLiaquat University of Medical and Health Sciences Jamshoro, Sindh, Pakistan
fJinnah Sindh Medical University, Karachi, Pakistan
gDow University of Health sciences, Karachi, Pakistan
hUniversity of Jordan, Amman, Jordan
iCorresponding Author: Wing Lam Ho, St George’s University School of Medicine, West Indies, Grenada
Manuscript submitted August 26, 2024, accepted November 21, 2024, published online December 31, 2024
Short title: New Onset Atrial Fibrillation in Sepsis
doi: https://doi.org/10.14740/cr1723
| Abstract | ▴Top |
Atrial fibrillation (AF) is a common arrhythmia in critically ill patients. The objective of this narrative review is to evaluate the characteristics of patients who develop new-onset atrial fibrillation (NOAF) because of sepsis, current management of NOAF in sepsis patients, special consideration in different populations that developed NOAF, health economic and quality of life of patients. We conducted a literature search on PubMed to find research related to NOAF, sepsis and critical illness. Nineteen studies were analyzed for risk factors and outcomes. The incidence rate ranges from 0.53% to 43.9% among these studies. There were numerous risk factors that had been reported from these articles. The most reported risk factors included advanced age, male sex, White race, and cardiovascular comorbidities. The management of septic patients is significantly challenging because of the unfavorable cardiovascular consequences and thromboembolic hazards associated with NOAF. There are comprehensive guidelines available for managing AF, but the effectiveness and safety of therapies in patients with sepsis are still uncertain. Various approaches for managing newly diagnosed AF have been explored. Sinus rhythm can be restored through either pharmacological or non-pharmacological intervention or combination of both. In addition, thromboembolism is a complication that can occur in patients with AF and can have a negative impact on the prognosis of sepsis patients. The use of anticoagulation to prevent stroke after NOAF in sepsis patients is still controversial. Extensive prospective investigations are required to have a deeper understanding of the necessity for anticoagulation following NOAF in sepsis. Beside the treatment of NOAF, early detection of NOAF in sepsis plays a critical role. The prompt initiation of rhythm control medication following a clinical diagnosis of AF can enhance cardiovascular outcomes and reduce mortality in patients with AF and cardiovascular risk factors. Additionally, NOAF in the intensive care unit can prolong hospital stays, increasing hospitalization costs and burdening the hospital. Therefore, preventing and managing NOAF effectively not only benefit the patients but also the hospital in financial aspect. Lastly, to address the existing gaps in knowledge, future research should focus on developing machine learning models that can accurately anticipate risks, establish long-term follow-up protocols, and create complete monitoring systems. The focus is on early intervention and personalized approaches to improve outcomes and quality of life.
Keywords: Sepsis; New-onset atrial fibrillation; Septic shock; Cardiac complications in sepsis; Arrhythmia in sepsis
| Introduction | ▴Top |
The definition of sepsis was initially established in 1992 through an agreement among prominent experts in the field of critical care. The definition of sepsis underwent its third change in 2016. It is now described as a life-threatening condition where the organs fail due to the host’s dysregulated response to infections, which ultimately can lead to death [1]. A subtype of sepsis, septic shock is characterized by circulatory, cellular, and metabolic instability, which is associated with a higher risk of mortality than sepsis itself [1]. Sepsis can lead to cytokine-induced lung damage, resulting in noncardiogenic pulmonary edema or acute respiratory distress syndrome. In addition, it can lead to acute renal injury, anemia, leukocytosis, neutropenia, thrombocytopenia, and disseminated intravascular coagulation [2]. Furthermore, the prevalence of new-onset atrial fibrillation (NOAF) is higher in sepsis patients, and up to 60% of septic patients develop myocardial depression [2, 3].
Atrial fibrillation (AF) is a common arrhythmia that frequently occurs in patients with sepsis [4]. It occurs in approximately one out of every seven individuals [3]. NOAF can be triggered by several factors such as systemic inflammation, upregulated stress hormones, autonomic dysfunction, and volume alterations which are all induced by sepsis [5]. This condition is linked to a higher likelihood of death during hospitalization and increased mortality rates in the intensive care unit [3]. In addition, NOAF in sepsis patients require longer hospitalization and advanced treatment with poor prognosis [3]. Despite all the poor outcomes of NOAF during sepsis, there is not enough information to guide its therapy, which poses considerable issues for sepsis patients. The aim of this review is to analyze the attributes of patients who develop NOAF due to sepsis, the current guideline of management, and suggested predictive models and methods for NOAF in sepsis patients.
| Methods | ▴Top |
To conduct a narrative review, we performed a comprehensive search on PubMed, encompassing all records from 2000 to 2024. The search utilized Medical Subject Headings (MeSH) and included the keywords AF, arrhythmia, sepsis, septicemia, and septic shock. We conducted a title and abstract screening to exclude studies outside the scope of our research, resulting in 157 articles being included. A full-text review of the remaining 157 articles further excluded 54 studies that did not meet the inclusion criteria for relevance or scope. Exclusion criteria were applied to omit studies focused on pre-existing AF, those unrelated to sepsis-related cardiac outcomes, and articles that were case reports, reviews, or not available in English. This helped narrow the scope to studies directly aligned with our research objective.
| Results | ▴Top |
After filtering out duplicates and journals that are irrelevant to our research query from the initial database search of 2,093 results, 157 articles were chosen for additional screening. At the end, 71 articles were chosen for analysis after two rounds of screening.
Pathophysiology of AF in sepsis
The pathogenesis of NOAF in sepsis may be more complicated than that of AF in non-critical patients. There are numerous proposed mechanisms that contribute to the development of NOAF during sepsis. For AF to occur, there are two prerequisites: 1) the development of a cardiac substrate that predisposes to abnormal heart rhythms in the atria; 2) the arrhythmogenic trigger (Fig. 1) [6-11]. Sepsis can lead to an elevation in inflammatory cytokines, free radicals, and oxidative stress, all of which play a crucial role in the body’s inflammatory reactions [7]. Inflammation can lead to accelerated heart structural and electrical remodeling, which fulfils the first requirement of developing NOAF [6]. All these physiological changes during sepsis result in a six-fold higher risk of developing NOAF [8]. Myocyte injury, excessive adrenergic stimulation or electrolyte derangements can be the trigger of NOAF in sepsis [6]. Furthermore, Aoki et al have suggested that sepsis induced shortening of action potential duration, which predisposes to NOAF, can be one of the mechanisms [9]. In addition, Shaver et al have proposed a theory to elucidate the disparity in the development of NOAF across sepsis patients. They hypothesized that certain patients carried intrinsic predispositions for developing AF, such as genetic disorders, and that these predispositions were unmasked after experiencing sepsis which caused the accompanying physiological alterations [10]. Furthermore, a recent study has identified four genes associated with neutrophil extracellular trap production in sepsis and AF. The study also showed that the generation of neutrophil extracellular traps in the atrium may have a role in the development of NOAF in sepsis [11]. Therefore, it is important to have a greater understanding of the fundamental pathophysiology of NOAF during sepsis. This will enable the discovery of new therapeutic targets and the implementation of treatment strategies that directly address the underlying causes.
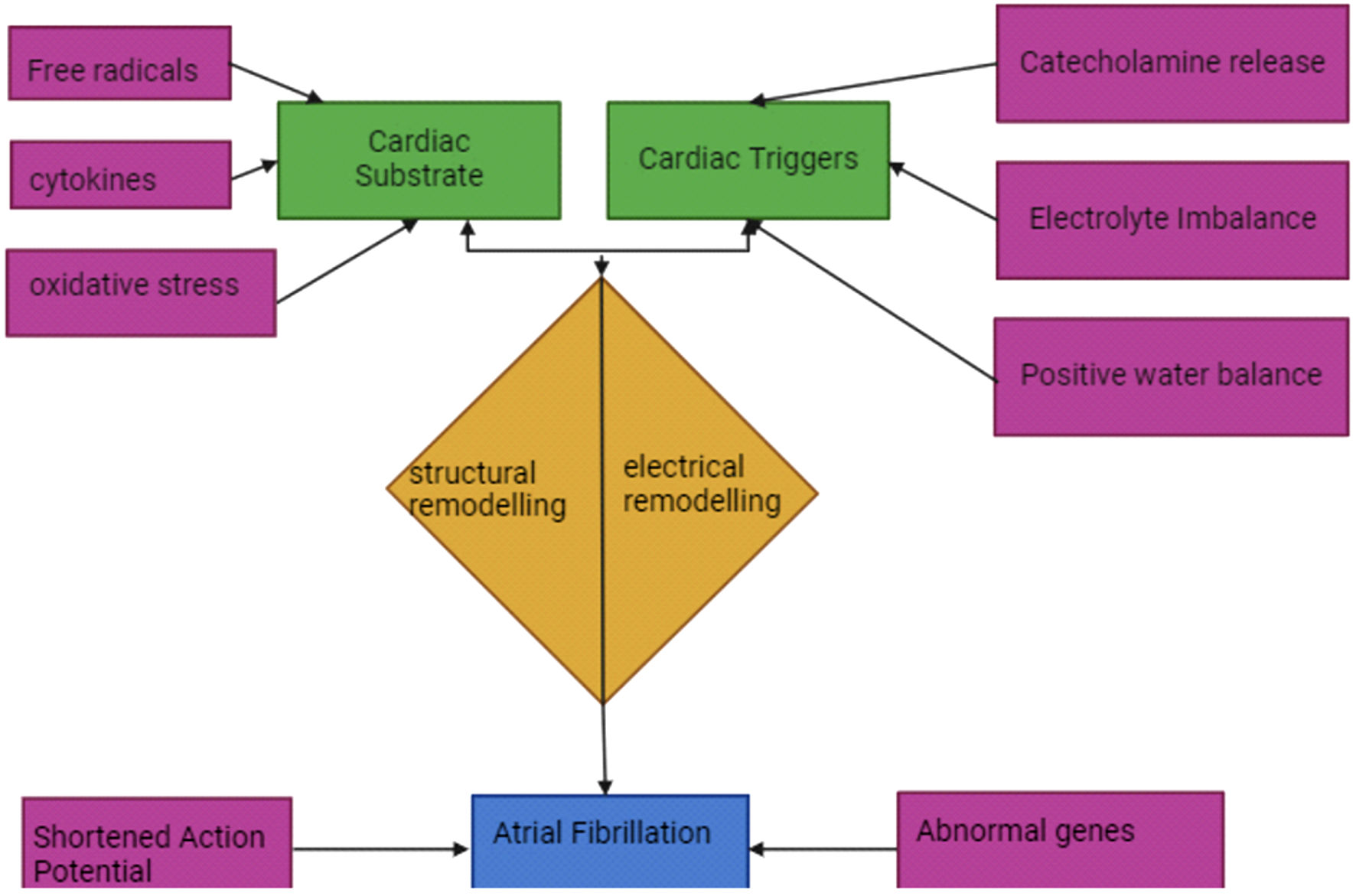 Click for large image | Figure 1. Proposed pathophysiology mechanisms of NOAF in sepsis. NOAF: new-onset atrial fibrillation. |
Risk factors for NOAF in sepsis
Classical risk factors of developing AF in general populations are advanced age, White race, cardiovascular comorbidities, postoperative setting, obesity, diabetes, and smoking [12]. Similarly, consistently reported risk factors for a higher likelihood of developing NOAF in critical illness also included advanced age, male sex, White race, and having comorbidities (Table 1) [10, 13-31]. In 15 out of 20 studies analyzed, advanced age had been associated with an increased incidence rate of NOAF in the intensive care unit (ICU) or sepsis patients, with statistical significance [10, 13-26]. There are four out of nine ICU studies that have shown a higher incidence rate of NOAF with sepsis vs. non-sepsis [10, 18, 19, 27]. Few studies had also shown that higher sequential organ failure assessment (SOFA) score is associated with increased risk of developing NOAF in sepsis patients [26, 28]. Shaver et al [10] and Makrygiannis et al [18] also reported that increased left atrial dimensions were associated with a higher incidence rate of NOAF in sepsis patients. Mechanical ventilation was also associated with higher incidence risk of NOAF [13, 24]. Cardiovascular comorbidities were the most common reported comorbidities among studies, which include hypertension, valvular disease, congestive heart failure (CHF), myocardial infarction (MI), and coronary artery disease (CAD) (Table 1) [10, 13-31]. However, Walkey et al have reported these comorbidities were not associated with increased risk of NOAF during sepsis [24, 25]. There were some other comorbidities that were reported to be associated with higher risk of developing NOAF in critical illness, which included chronic obstructive pulmonary disease (COPD), cerebrovascular disease, and diabetes (Table 1) [10, 13-31].
 Click to view | Table 1. Study Characteristics, Risk Factors, and Outcomes for NOAF in Critical Illness |
Clinical presentation and diagnosis
Diagnosing NOAF in septic patients involves recognizing its clinical signs, using tools like electrocardiogram (ECG), continuous monitoring, and addressing challenges posed by critical illness [6]. Research by Bosch et al had indicated that any organ dysfunction, especially acute cardiac, respiratory, and renal failures, correlates closely with AF development compared to other organ dysfunctions [32, 33]. Bosch et al [32] had also reported that the most frequent and strongest risk factors were associated with the sepsis event and treatment rather than cardiovascular conditions. Surprisingly, this meta-analysis found that some risk factors that caused AF were not associated with the development of NOAF during sepsis like CAD, diabetes mellitus, hypertension and valvular disease. This may indicate that focusing on sepsis treatment may improve patient outcomes, and AF may be prevented or resolved more quickly [32]. Sepsis patients face increased risks of cardiovascular complications, including MI [34-36], heart failure [34, 37, 38], new-onset AF, and stroke [25, 36], alongside increased mortality rates. NOAF in our study was identified as a newly detected episode of AF in patients with no prior history. Various methods for detecting AF from ECG signals have been explored, with some focusing on analyzing atrial and ventricular activities and others employing techniques like wavelet transforms and machine learning. Algorithms relying solely on P wave absence often struggle with noise and baseline signal variations, compromising accuracy, particularly in the presence of premature beats [39]. To address these challenges, a novel AF detection algorithm integrating long-term ECG analysis, premature beat detection, and AF identification in critically ill patients is proposed [39]. Procalcitonin (PCT), as a marker of inflammation and tissue injury in bacterial sepsis, aids in diagnosing and prognosticating infections [40]. Although elevated PCT levels are observed in chronic kidney disease patients on hemodialysis (CKD5-HD), its association with inflammatory cardiac conditions like AF remains unclear [40-44]. Studies suggest that systemic inflammation, indicated by elevated C-reactive protein (CRP) levels, plays a role in NOAF development, particularly in critically ill patients with septic shock [45]. Higher CRP levels and white blood cell counts during NOAF episodes have been noted, underscoring the potential link between inflammation and AF maintenance [45-47]. Future prospective studies are essential to validate these findings and explore the complex interplay between inflammation, AF, and critical illness.
Prognostic implications of AF in sepsis
NOAF is a significant complication in sepsis, impacting both short-term and long-term outcomes. It frequently occurs in critically ill patients, particularly those with sepsis and significantly impacts their prognosis [5]. NOAF is associated with heightened risks of mortality and arterial thromboembolic events. Transesophageal echocardiography (TEE) identifies abnormalities such as left ventricular dysfunction and dysfunction of the left atrium/left atrial appendage (LA/LAA), which increase the likelihood of thromboembolic events due to blood stasis and dense spontaneous echo contrast [48]. Bedside echocardiography is thus valuable for assessing cardiovascular risk in these patients [48]. NOAF is an independent risk factor for mortality in septic shock, MI, and heart failure patients [24]. It complicates the clinical course of sepsis by worsening hemodynamic status, as it can lead to rapid heart rate (HR), irregular rhythms, and loss of atrial systole. This exacerbates cardiovascular instability and complicates treatment during sepsis or septic shock, potentially leading to acute heart failure [25, 49]. Moreover, atrial stasis and sepsis-related coagulopathy increase the risk of systemic embolization and ischemic stroke [24, 25, 49]. Restoration of SR improves hemodynamic stability by enhancing diastolic filling and left ventricular systolic performance. This favorable outcome contrasts with patients who fail to regain SR, highlighting the therapeutic significance of managing NOAF promptly in septic patients [26, 50]. In summary, NOAF poses significant challenges in managing septic patients due to its adverse cardiovascular effects and thromboembolic risks. Prompt intervention to restore SR can mitigate these complications and improve patient outcomes, underscoring the critical role of early detection and management strategies tailored for sepsis-associated NOAF.
Management strategies: rate control vs. rhythm control
There are many ways to manage AF in sepsis. In this study, we have summarized various managements (Fig. 2) [51-61]. Elevated circulating catecholamine levels in sepsis may raise the possibility of fast atrioventricular-nodal conduction in AF, which could shorten the diastolic filling time and raise the risk of hemodynamic instability [62, 63]. Therefore, in patients who do not require emergency electric cardioversion, practice guidelines recommend using medications to lower HR in AF with rapid ventricular response (RVR) [64]. An HR of more than 110 beats per minute was the upper limit of HR control in previous trials, and this was the definition of AF with RVR [65, 66].
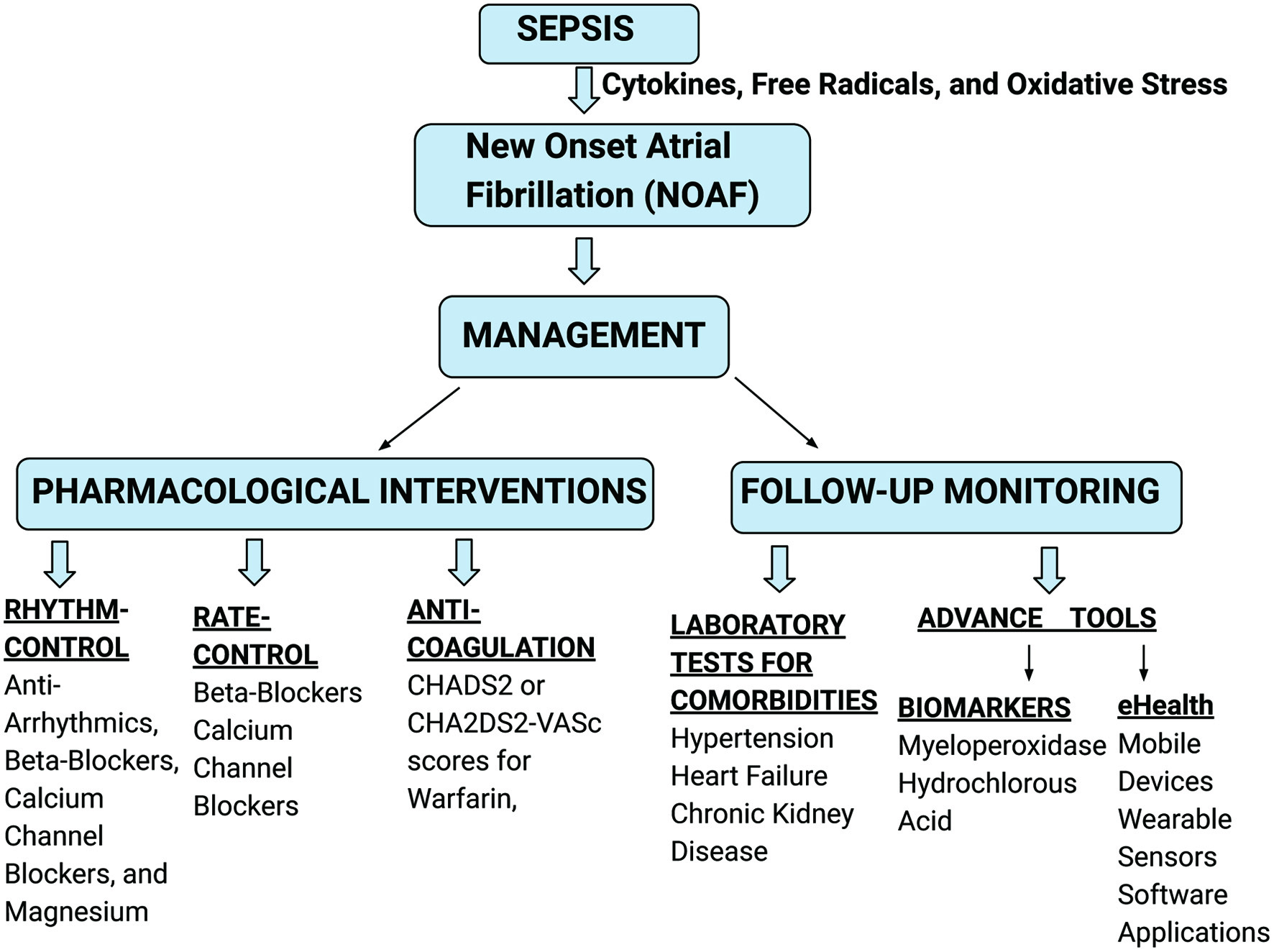 Click for large image | Figure 2. Various managements for NOAF in sepsis. NOAF: new-onset atrial fibrillation. |
There are extensive recommendations for addressing AF in both the community and acute settings [67, 68]. However, the safety and efficacy of treatments in critically ill patients remain unclear [69]. Variable treatment strategies for NOAF have been considered. There are two therapeutic strategies which are generally utilized: rate control and rhythm control. The rate control therapy mostly uses beta-blockers and calcium channel blockers. While cardioversion, antiarrhythmics, and catheter ablation can achieve rhythm control [51].
Amiodarone, beta-blockers, calcium channel blockers, and magnesium provide equal rates of sustained rhythm control in NOAF patients [52]. The rate-control drugs include beta-blockers (metoprolol and esmolol) and calcium channel blockers (diltiazem and verapamil). With up to 37% of patients experiencing hemodynamic compromise in conjunction with AF, those with new-onset AF during critical illness may benefit most from a rapid reduction in HR [52]. However, they appear to be beneficial in rhythm control. Beta-blockers and magnesium are a bit more effective in rhythm control than calcium channel blockers and amiodarone [53]. The safety of beta-blockers and calcium antagonists is under question, particularly in critically ill individuals. This is because of the likelihood of hypotensive side effects. Amiodarone and digitalis are frequently administered to severely ill patients [51].
Gillmann et al found that in 23% of individuals, electrical cardioversion followed by medicine resulted in a conversion to sinus rhythm. Amiodarone was more effective for cardioversion [51]. Amiodarone can be very effective in controlling rate and rhythm after beta-blocker therapy [54]. Dofetilide (DF) and sotalol (SL) are class III antiarrhythmics with minimal non-cardiac side effects, often studied in cases of NOAF in sepsis. Dofetilide blocks the rapid delayed rectifier potassium current (IKr), extending action potential duration and QT interval by delaying repolarization, which prolongs the refractory periods of the His-Purkinje system and ventricles. Sotalol, on the other hand, exhibits both class II and III antiarrhythmic effects by non-selective beta blockade and blocking rapid component of delayed rectifier potassium channels, respectively [55]. Sotalol has been shown to cause significant QT prolongation even at low doses and serum concentrations. Research indicates a strong correlation between serum sotalol levels and QTc prolongation, regardless of the administration route (oral or intravenous), with similar effects observed across equivalent concentrations. This underscores the need for careful QT interval monitoring, particularly when initiating or titrating doses, to mitigate the risk of torsades de pointes and other arrhythmias [56]. Digoxin and DC cardioversion may be less successful than other therapies in critically sick individuals with NOAF [70]. In individuals with AF, rate control is just as critical as rhythm control. For critically ill NOAF patients treated with beta-blockers or calcium channel blockers, rate control may lead to rhythm control by permitting spontaneous cardioversion. In a study, postoperative AF treatment strategies led to similar hospitalization days, complication rates, and low persistent fibrillation rates. However, neither treatment showed a net therapeutic benefit over the other [71].
Non-pharmacological interventions
According to European guidelines, cardioversion is advised for critically ill patients with unstable AF [25]. The goal of cardioversion is to improve left ventricular filling and reduce cardiac metabolic demand to restore hemodynamics. In a prior series of critically ill NOAF patients, 23-32% of patients had successful electrical cardioversion [72, 73]. Amiodarone may have helped to maintain sinus rhythm stability following the quick success of cardioversion, as previously noted by Sticherling et al in cardiological settings [74]. The potential risk of cardioversion-related arterial thrombotic events reported in outpatients is highlighted by the arterial thrombotic events that happened in the successful cardioversion group [75].
Moreover, compared to medication therapy, catheter ablation lowers all-cause mortality in patients with AF and heart failure [76]. The main objectives of catheter ablation are to enhance quality of life and get rid of AF symptoms. Catheter ablation is not usually chosen as a first-line treatment after balancing consequences such as atrioesophageal fistula, cardiac tamponade, phrenic nerve injury, esophageal injury, and pulmonary vein contracture [77].
Anticoagulation in sepsis-associated AF
The thromboembolic phenomenon is one of the complications of AF, and it can worsen the prognosis in sepsis patients. Although warfarin has shown a promising role in primary AF, no clear significant findings are available to support this notion in secondary AF, especially in sepsis [78]. Variable risks and benefits have been reported so far. Anticoagulation therapy used to prevent ischemic stroke is associated with various side effects like bleeding and heparin-induced thrombocytopenia [78]. A study demonstrated when anticoagulation is used in AF patients with sepsis. They observed that it did not lower the ischemic stroke risk. However, they were linked with increased bleeding risks [79]. Darwish et al found higher adverse events with warfarin and enoxaparin but not with unfractionated heparin [78]. However, studies have shown the beneficial aspects of anticoagulation in AF management are greater than antiplatelet therapy against cerebral infarction [79]. Zusman et al observed that warfarin usage was a significant predictor of stroke-free survival [58]. Milika et al discovered that patients who received anticoagulant medication upon hospital discharge had a considerably lower incidence of ischemic stroke [59].
CHADS2 or CHA2DS2-VASc scores can be used to screen patients for the risk of central nervous system (CNS) complications [12, 80, 81]. Patients with NOAF that lasts for more than 48 h and are at risk of CNS complications should receive anticoagulant medication along with rhythm or rate control therapy [68]. Parenteral anticoagulation should be administered with caution in patients with AF during sepsis episodes due to the significant risk of bleeding described in previous trials [82-84]. The American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines recommend warfarin for patients with transient NOAF having the CHA2DS2-VASc score greater or equal to 2 [64]. These guidelines recommend addressing the underlying cause, considering the patient’s risk profile, and AF duration while deciding on anticoagulant medication. Considering the limited long-term evidence, it is advised that these patients should be carefully followed up [64]. Further, clinicians should carefully monitor their patients and be ready to start more AF medication, consider giving their patients more frequent doses, or start continuous infusions if their HR response is transient.
Markers for screening and detection of NOAF in sepsis
AF is one of the cardiovascular diseases with an increasing incidence globally. It is linked to higher rates of morbidity and mortality. The hemodynamic stability determines if rhythm control therapy is required [85]. In addition to having lower levels of hemoglobin, triglycerides, total and low-density lipoprotein cholesterol, and creatinine, patients with AF are found to have considerably higher levels of fasting blood sugar. Additionally, there was a higher likelihood of hypertension, CHF, chronic kidney disease (CKD), and cerebrovascular accidents among AF patients [86]. New data show that early rhythm control therapy, initiated after a recent clinical diagnosis of AF, can improve cardiovascular outcomes and mortality in patients with AF and cardiovascular risk factors. These findings will change the concept and practice of rhythm management. There has also been increasing focus on cognitive function assessment in patients with AF for early detection and prevention of cognitive impairment and its negative impact on treatment compliance [60]. These new data call for better integration of AF screening and early rhythm control in clinical care [87].
AF has a great effect on various physiological pathways, and thus a huge number of biomarkers can be assessed, which likely provide the clinician with important diagnostic and prognostic values. Natriuretic peptide (NP), oxidative stress, fibrosis, inflammation, coagulation, physiological pathways of microRNA (miRNA), myocardial stretch, and injury are the currently used biomarkers that offer the potential for guiding therapy [60]. Furthermore, most recent studies have reported myeloperoxidase and hydrochlorous acid level were higher in NOAF compared to non-NOAF group in sepsis patients, which indicates that the combination of these two markers may be an effective tool to predict the risk of developing NOAF in sepsis [88]. In addition, Beyls et al have analyzed both left and right atrium reservoir phase by using transthoracic echocardiogram. They have reported that bi-atrium dysfunction can predict the development of NOAF in sepsis [89]. Hence, variable markers can be utilized for screening and detection of NOAF. The use of economical, easily accessible markers may accelerate the process of accurate diagnosis, which makes the treatment of AF more effective [3].
Advancement in monitoring tools of AF in sepsis
Detecting NOAF in ICU patients with sepsis is crucial, as early identification can prompt timely interventions to mitigate risks like stroke and worsening cardiac function. However, this detection is challenging because sepsis often leads to fluctuating HRs and irregular rhythms, which can mask AF episodes. Additionally, ICU environments are prone to noise artifacts in ECG data, caused by patient movement, monitoring equipment, and frequent medical procedures, which complicate accurate AF detection. This pain point has paved the way for innovative and advanced detection technologies to be developed. A novel two-step algorithm using HR variability, Poincare plots, and P-wave characteristics was developed and tested on 198 sepsis patients (4,209 h of ECG data), achieving 100% sensitivity, 98% specificity, and 98.99% overall accuracy, outperforming existing methods by minimizing false positives due to premature atrial contractions (PACs)/premature ventricular contractions (PVCs) [90]. When comparing with traditional methods, another automated AF detection algorithm for ICU patients with sepsis showed superior performance over traditional methods (International Classification of Diseases, Ninth Revision (ICD-9) coding and nurse charting) with a sensitivity of 92% (95% confidence interval (CI): 74-99%), specificity of 96% (95% CI: 80-100%), and overall accuracy of 94% (95% CI: 83-99%), achieving a median detection time 30 min faster than manual detection [91].
An artificial intelligence (AI)-based ICU monitoring system for sepsis detection demonstrated high accuracy, with the support vector classifier (SVC) achieving area under the receiver operating characteristic curve (AUROC) of 0.92 and area under the precision-recall curve (AUPRC) 0.90, strong recall 0.86, precision 0.80, and specificity 0.80, while logistic regression reached AUROC of 0.91 and precision 0.92; key predictive features included pulse arrival time (PAT), heart rate variability (HRV), and respiratory parameters, with significant metrics like NN50 and pNN50 showing strong associations with sepsis risk, and linear models outperformed tree-based methods like XGB in leveraging waveform-derived features [61]. Shifting from real-time detection to predictive analytics, a machine learning-based AF prediction algorithm for ICU sepsis patients, utilizing ECG-derived HRV features and achieving 80% sensitivity, 100% specificity, and 90% accuracy, with the ability to predict AF up to 10 min before onset [91]. This extends beyond detection to proactively predict AF episodes, which offers ICU teams the opportunity for pre-emptive intervention. Ultimately, these technological advancements allow timely and reliable tools to improve patient outcomes, marking a shift from reactive to proactive AF management in sepsis patients.
Special considerations in specific populations
For NOAF in sepsis patients, gender also plays a role. A study has been stated that male gender was associated with an increased incidence of AF during sepsis [5, 13, 17, 23, 25]. This gender disparity was magnified by a large-scale retrospective cohort study using the National Inpatient Sample databases (2010 - 2014), which also reported sepsis-AF cohort had a higher percentage of male patients (51.1% vs. 47.5%; P < 0.001) compared to those without AF [17]. However, despite the higher prevalence in males, female gender was identified as one of the greatest predictors of mortality within the sepsis-AF cohort [17]. This suggests that while males are more likely to develop AF during sepsis, females with AF may have a higher risk of adverse outcomes and mortality, highlighting a significant gender disparity in the prognosis of sepsis-related AF. This gender-specific risk might be due to differences in comorbidities, response to treatment, or physiological differences between males and females.
Regarding the last factor, hormonal differences, such as the influence of estrogen, can also affect cardiovascular function and immune response. Estrogen has a protective effect against AF by modulating ion channel activities and reducing fibrosis, particularly through the transforming growth factor (TGF)b/Smad3 pathway; conversely, testosterone’s pro-arrhythmic properties, mediated by enhanced adrenergic activity, align with the higher male prevalence [92]. Furthermore, estrogen also increases L-type calcium channel expression and activity in cardiomyocytes, prolonging action potential duration in atrial cells, which exerts an anti-arrhythmic effect by reducing re-entrant circuits, thereby contributing to the lower incidence of AF in women compared to men [92]. Moreover, the same estrogenic effects on calcium handling that lower AF incidence can lead to worse symptoms and higher risks of adverse events once AF occurs, as increased intracellular calcium from enhanced L-type calcium channel activity promotes atrial remodeling, including fibrosis and enlargement, exacerbating AF symptoms and increasing susceptibility to stroke and heart failure [17]. Structural differences were noted, with women showing more significant fibrotic remodeling in persistent AF [92]. Lastly, females may have a different inflammatory response to sepsis, which can influence the severity and outcomes of the condition. Additionally, differences in cardiac structure and function between genders can affect how AF impacts heart health. However, it is still unclear that how much of sex differences are caused by sex hormones and whether the lack of estrogen during postmenopausal is the primary cause of changes in comorbidities conditions which indirectly increase the risk of developing AF [92].
NOAF in sepsis is also notably prevalent among the elderly, with its incidence increasing with age [10, 13-26]. Management of AF in elderly patients is complex due to multiple comorbidities like hypertension, diabetes, CAD, heart failure, valvular diseases, atrial septal defects, conditions elevating right ventricular afterload (e.g., pulmonary emboli, COPD, sleep apnea), increased body mass index (BMI), thyroid disease, metabolic syndrome, CKD, surgical or infection-related stress, and atrial inflammation [13, 93]. These comorbidities are often correlated with sepsis as they can weaken the immune system, making patients more susceptible to severe infections and systemic inflammatory responses, thereby increasing the risk of sepsis or septic shock [93]. Consequently, these conditions often necessitate polypharmacy (defined as having five or more active prescriptions), which can lead to significant issues like medication non-adherence, as well as bleeding risks and heparin-induced thrombocytopenia, particularly in elderly patients with a CHADS2 score at 2 or more in the setting of sepsis, who are on anticoagulation therapy [94, 95]. Therefore, considering the high prevalence and complexity of AF in elderly patients, various management approached may be considered.
Health economic and quality of life aspects
The management of NOAF in sepsis patients presents significant economic challenges due to the need for intensive monitoring and treatment. Fernando et al had demonstrated through their retrospective analysis that new-onset AF in ICU patients led to a 9% cost increase and longer stays, with rhythm control strategies driving costs even higher (24% increase) [16]. Complementing this, Lin et al revealed a striking rise in the national trends in AF prevalence among long-term ventilated patients, from 14.63% in 2008 to 24.43% in 2014 [96]. This upward trajectory in AF prevalence was accompanied by a 4% increase in hospitalization costs and a 2% extension in length of stay (LOS) [96]. However, the narrative takes an unexpected turn. Despite the rising tide of AF cases, this study observed annual decreases in both LOS (-1%) and hospitalization costs (-4%) [96]. Such a counterintuitive trend suggests a sophisticated dynamic between disease prevalence and management strategies, which invites us to consider the possibility of significant advancements in AF management practices, capable of mitigating the economic and logistical challenges posed by increasing prevalence. The temporal dimension of AF management emerges as a critical factor in the study of Azahar et al [97]. Their findings, derived from a Malaysian medical center, illustrate the profound impact of early intervention, with a striking median cost reduction of Malaysian Ringgit (MYR) 2,839.73 (approximately $680) per AF-related stroke case, which highlights the axiom that timely action not only saves lives but also conserves valuable healthcare resources [97]. As we navigate this multifaceted landscape, the imperative for healthcare providers becomes clear: to craft management protocols that deftly balance the immediacy of cost considerations with the longevity of patient outcomes. The judicious implementation of early interventions, coupled with a discerning approach to anticoagulant selection, holds the potential to optimize both clinical efficacy and economic sustainability.
Patients with sepsis who develop AF face significantly worse outcomes, with statistically significant increased risks of mortality for sepsis patients with AF [3, 13, 14, 16, 17, 19, 21-23, 25, 26, 29, 31, 98]. Steinberg et al conducted a study, which suggested that higher AF symptom scores are associated with worse physical function, poorer general health perceptions, and higher levels of depression [99]. This reinforces the notion that AF profoundly impacts patients’ overall quality of life and mental health, not just their cardiovascular health. These findings support the need for comprehensive care approaches that address both the physical and psychological aspects of AF, especially in high-risk populations like those with sepsis. It also stresses the importance of incorporating patient-reported outcomes (PROs) into clinical practice and decisions management to better understand and manage the multifaceted impacts of AF on patients. To illustrate, a study reported that patients with NOAF who understood their oral anticoagulation and rhythm control options had higher odds of participating in shared decision-making (OR: 2.54, CI: 1.75 - 3.68; and OR: 2.36, CI: 1.50 - 3.71, respectively), which highlights the importance of patient education in improving engagement and potentially outcomes [100]. In essence, the evidence strongly suggests that managing AF effectively requires a holistic approach that includes both medical treatment and patient education to enhance shared decision-making and overall patient well-being, beginning with an emphasis on PROs to ensure a thorough understanding of the patient’s physical, mental, and emotional well-being.
Current gaps in knowledge and future research directions
There were limitations in this study that are vital to mention. Firstly, there were several papers relevant to the topic that we could not gain access to via the known databases. Secondly, other papers that could have had elevated significance in our research were written in languages other than English and were therefore automatically excluded from the review. Moreover, some papers were not included in the study as they were preprints under review by their publishing journals. The treatment approach for patients with AF was not standardized in all studies, potentially introducing unidentified bias. Furthermore, there might have been missed AF episodes that were not documented, particularly short-term, self-resolving episodes [14]. Another limitation that should be addressed is the likely overrepresentation of high-income groups compared to middle-income and low-income groups [101]. Several papers were retrospective observational studies, which results in a higher risk of sampling errors and selection bias [102]. To gain a more comprehensive understanding of the long-term impact of AF, additional factors such as quality of life and extended patient follow-up should be taken into consideration. A study that developed and validated a machine learning model capable of approximately anticipating the 28-day mortality risk in septic patients with AF has the potential to enhance clinical practice [15].
| Conclusions | ▴Top |
Sepsis-related NOAF is a serious condition linked to higher death rates, thromboembolic events, and extended hospital stays. Because of heart remodeling, autonomic dysfunction, and systemic inflammation, it implicates major diagnostic and treatment challenges. Advanced age, male sex, cardiovascular comorbidities, and higher SOFA scores are major risk factors of NOAF in sepsis. The management of NOAF in sepsis carries significant financial issues since it results in higher expenses and longer hospital stays. Despite this, improvements in management techniques and early intervention offer promise for reducing these difficulties. Patient outcomes are markedly worsened by NOAF, impacting both physical and mental well-being. Holistic care requires the integration of PROs into therapeutic practice. Although safety issues still exist, management techniques include rate and rhythm regulation with beta-blockers, calcium channel blockers, amiodarone, and magnesium. Besides its benefits, anticoagulation medication must be carefully considered because of the danger of bleeding. Due to advance technology these days, wearable technology and AI-powered systems can be used for monitoring that enable early detection and integrated care, potentially lowering hospitalization and mortality rates. Treatments may be altered in different populations. Tailored treatment techniques are necessary for older patients and people of different genders; non-vitamin K antagonist oral anticoagulants (NOACs) are more beneficial than warfarin. To fill in the current knowledge gaps, future research should concentrate on machine learning models for risk prediction, long-term follow-up, and comprehensive monitoring systems. A thorough, multidisciplinary strategy that achieves a balance between patient-centered care and financial concerns is needed to manage NOAF in sepsis. Early intervention and individualized approaches are prioritized to enhance outcomes and quality of life.
Acknowledgments
None to declare.
Financial Disclosure
The authors declare that they have no financial relationship with any commercial entity that has an interest in the subject of this manuscript.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Author Contributions
Wing Lam Ho wrote the manuscript parts of the Abstract, Introduction, Methods, Conclusions, and the section of “Risk factors for NOAF in sepsis”. Sara Izhar wrote the Pathophysiology part, including Figure 1. Kajal Kumari wrote the sections of “Clinical presentation and diagnosis” and “Prognostic implications of AF in sepsis”. Meena Bai and Muhammad Umais wrote the sections of “Management strategies: rate control vs. rhythm control”, “Non-pharmacological interventions”, and “Anticoagulation in sepsis-associated AF” together. Rabia Asrar and Muhammad Umais wrote the sections of “Markers for screening and detection of NOAF in sepsis” and “Advancement in monitoring tools of AF in sepsis”. Ngoc Bao Dang wrote the sections of “Special considerations in specific populations” and “Health economic and quality of life aspects”. Toleen Haddad wrote the section of “Current gaps in knowledge and future research directions”. Muhammad Ali Muzammil supervised and mentored the authors for this work. All of the authors did the literacy search, wrote and edited the manuscript together.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Srzic I, Nesek Adam V, Tunjic Pejak D. Sepsis definition: what's new in the treatment guidelines. Acta Clin Croat. 2022;61(Suppl 1):67-72.
doi pubmed - Font MD, Thyagarajan B, Khanna AK. Sepsis and Septic Shock - Basics of diagnosis, pathophysiology and clinical decision making. Med Clin North Am. 2020;104(4):573-585.
doi pubmed - Corica B, Romiti GF, Basili S, Proietti M. Prevalence of new-onset atrial fibrillation and associated outcomes in patients with sepsis: a systematic review and meta-analysis. J Pers Med. 2022;12(4):547.
doi pubmed - Aibar J, Schulman S. New-onset atrial fibrillation in sepsis: a narrative review. Semin Thromb Hemost. 2021;47(1):18-25.
doi pubmed - Kuipers S, Klein Klouwenberg PM, Cremer OL. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit Care. 2014;18(6):688.
doi pubmed - Bosch NA, Cimini J, Walkey AJ. Atrial fibrillation in the ICU. Chest. 2018;154(6):1424-1434.
doi pubmed - Induruwa I, Hennebry E, Hennebry J, Thakur M, Warburton EA, Khadjooi K. Sepsis-driven atrial fibrillation and ischaemic stroke. Is there enough evidence to recommend anticoagulation? Eur J Intern Med. 2022;98:32-36.
doi pubmed - Walkey AJ, McManus D. When rhythm changes cause the blues: new-onset atrial fibrillation during Sepsis. Am J Respir Crit Care Med. 2017;195(2):152-154.
doi pubmed - Aoki Y, Hatakeyama N, Yamamoto S, Kinoshita H, Matsuda N, Hattori Y, Yamazaki M. Role of ion channels in sepsis-induced atrial tachyarrhythmias in guinea pigs. Br J Pharmacol. 2012;166(1):390-400.
doi pubmed - Shaver CM, Chen W, Janz DR, May AK, Darbar D, Bernard GR, Bastarache JA, et al. Atrial Fibrillation Is an Independent Predictor of Mortality in Critically Ill Patients. Crit Care Med. 2015;43(10):2104-2111.
doi pubmed - Xiang J, Cao J, Wang X, Shao S, Huang J, Zhang L, Tang B. Neutrophil extracellular traps and neutrophil extracellular traps-related genes are involved in new-onset atrial fibrillation in LPS-induced sepsis. Int Immunopharmacol. 2024;138:112550.
doi pubmed - Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379(9816):648-661.
doi pubmed - Liu YW, Wang YF, Chen Y, Dong R, Li S, Peng JM, Liufu R, et al. A nationwide study on new onset atrial fibrillation risk factors and its association with hospital mortality in sepsis patients. Sci Rep. 2024;14(1):12206.
doi pubmed - Paula SB, Oliveira A, Melo ESJ, Simoes AF, Goncalves-Pereira J. Atrial Fibrillation in Critically Ill Patients: Incidence and Outcomes. Cureus. 2024;16(2):e55150.
doi pubmed - Wang W, Dong Y, Zhang Q, Gao H. Atrial fibrillation is not an independent determinant of 28-day mortality among critically III sepsis patients. BMC Anesthesiol. 2023;23(1):336.
doi pubmed - Fernando SM, Mathew R, Hibbert B, Rochwerg B, Munshi L, Walkey AJ, Moller MH, et al. New-onset atrial fibrillation and associated outcomes and resource use among critically ill adults-a multicenter retrospective cohort study. Crit Care. 2020;24(1):15.
doi pubmed - Desai R, Hanna B, Singh S, Omar A, Deshmukh A, Kumar G, Foreman MG, et al. Trends and outcomes in sepsis hospitalizations with and without atrial fibrillation: a nationwide inpatient analysis. Crit Care Med. 2019;47(8):e630-e638.
doi pubmed - Makrygiannis SS, Rizikou D, Patsourakos NG, Lampakis M, Margariti A, Ampartzidou OS, Sakellaridis K, et al. New-onset atrial fibrillation and clinical outcome in non-cardiac intensive care unit patients. Aust Crit Care. 2018;31(5):274-277.
doi pubmed - Moss TJ, Calland JF, Enfield KB, Gomez-Manjarres DC, Ruminski C, DiMarco JP, Lake DE, et al. New-onset atrial fibrillation in the critically ill. Crit Care Med. 2017;45(5):790-797.
doi pubmed - Guenancia C, Binquet C, Laurent G, Vinault S, Bruyere R, Prin S, Pavon A, et al. Incidence and Predictors of New-Onset Atrial Fibrillation in Septic Shock Patients in a Medical ICU: Data from 7-Day Holter ECG Monitoring. PLoS One. 2015;10(5):e0127168.
doi pubmed - Wells GL, Morris PE. Incidence and prognosis of atrial fibrillation in patients with sepsis. Cardiol Res. 2011;2(6):293-297.
doi pubmed - Chen AY, Sokol SS, Kress JP, Lat I. New-onset atrial fibrillation is an independent predictor of mortality in medical intensive care unit patients. Ann Pharmacother. 2015;49(5):523-527.
doi pubmed - Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146(5):1187-1195.
doi pubmed - Walkey AJ, Greiner MA, Heckbert SR, Jensen PN, Piccini JP, Sinner MF, Curtis LH, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949-955.e943.
doi pubmed - Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306(20):2248-2254.
doi pubmed - Meierhenrich R, Steinhilber E, Eggermann C, Weiss M, Voglic S, Bogelein D, Gauss A, et al. Incidence and prognostic impact of new-onset atrial fibrillation in patients with septic shock: a prospective observational study. Crit Care. 2010;14(3):R108.
doi pubmed - Wetterslev M, Hylander Moller M, Granholm A, Hassager C, Haase N, Lange T, Myatra SN, et al. Atrial Fibrillation (AFIB) in the ICU: incidence, risk factors, and outcomes: the international AFIB-ICU cohort study. Crit Care Med. 2023;51(9):1124-1137.
doi pubmed - Steinberg I, Brogi E, Pratali L, Trunfio D, Giuliano G, Bignami E, Forfori F. Atrial fibrillation in patients with septic shock: a one-year observational pilot study. Turk J Anaesthesiol Reanim. 2019;47(3):213-219.
doi pubmed - Savaie M, Sheikhi Y, Baghbanian R, Soltani F, Amiri F, Hesam S. Epidemiology, risk factors, and outcome of cardiac dysrhythmias in a noncardiac intensive care unit. SAGE Open Nurs. 2023;9:23779608231160932.
doi pubmed - Launey Y, Lasocki S, Asehnoune K, Gaudriot B, Chassier C, Cinotti R, Maguet PL, et al. Impact of low-dose hydrocortisone on the incidence of atrial fibrillation in patients with septic shock: a propensity score-inverse probability of treatment weighting cohort study. J Intensive Care Med. 2019;34(3):238-244.
doi pubmed - Liu WC, Lin WY, Lin CS, Huang HB, Lin TC, Cheng SM, Yang SP, et al. Prognostic impact of restored sinus rhythm in patients with sepsis and new-onset atrial fibrillation. Crit Care. 2016;20(1):373.
doi pubmed - Bosch NA, Cohen DM, Walkey AJ. Risk factors for new-onset atrial fibrillation in patients with sepsis: a systematic review and meta-analysis. Crit Care Med. 2019;47(2):280-287.
doi pubmed - Bedford JP, Harford M, Petrinic T, Young JD, Watkinson PJ. Risk factors for new-onset atrial fibrillation on the general adult ICU: A systematic review. J Crit Care. 2019;53:169-175.
doi pubmed - Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116(7):793-802.
doi pubmed - Smilowitz NR, Gupta N, Guo Y, Bangalore S. Comparison of outcomes of patients with sepsis with versus without acute myocardial infarction and comparison of invasive versus noninvasive management of the patients with infarction. Am J Cardiol. 2016;117(7):1065-1071.
doi pubmed - Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611-2618.
doi pubmed - Beesley SJ, Weber G, Sarge T, Nikravan S, Grissom CK, Lanspa MJ, Shahul S, et al. Septic cardiomyopathy. Crit Care Med. 2018;46(4):625-634.
doi pubmed - Sato R, Kuriyama A, Takada T, Nasu M, Luthe SK. Prevalence and risk factors of sepsis-induced cardiomyopathy: a retrospective cohort study. Medicine (Baltimore). 2016;95(39):e5031.
doi pubmed - Bashar SK, Ding E, Albuquerque D, Winter M, Binici S, Walkey AJ, McManus DD, et al. Atrial fibrillation detection in ICU patients: a pilot study on MIMIC III Data. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:298-301.
doi pubmed - Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008;36(3):941-952.
doi pubmed - El Haddad H, Chaftari AM, Hachem R, Chaftari P, Raad II. Biomarkers of sepsis and bloodstream infections: the role of procalcitonin and proadrenomedullin with emphasis in patients with cancer. Clin Infect Dis. 2018;67(6):971-977.
doi pubmed - Bloos F, Trips E, Nierhaus A, Briegel J, Heyland DK, Jaschinski U, Moerer O, et al. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Intern Med. 2016;176(9):1266-1276.
doi pubmed - Dahaba AA, Rehak PH, List WF. Procalcitonin and C-reactive protein plasma concentrations in nonseptic uremic patients undergoing hemodialysis. Intensive Care Med. 2003;29(4):579-583.
doi pubmed - Herget-Rosenthal S, Marggraf G, Pietruck F, Husing J, Strupat M, Philipp T, Kribben A. Procalcitonin for accurate detection of infection in haemodialysis. Nephrol Dial Transplant. 2001;16(5):975-979.
doi pubmed - Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104(24):2886-2891.
doi pubmed - Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50(21):2021-2028.
doi pubmed - Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108(24):3006-3010.
doi pubmed - Di Biase L, Natale A, Romero J. Thrombogenic and arrhythmogenic roles of the left atrial appendage in atrial fibrillation. Circulation. 2018;138(18):2036-2050.
doi pubmed - Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920-2925.
doi pubmed - Viswanathan K, Daniak SM, Salomone K, Kiely T, Patel U, Converso K, Manning WJ, et al. Effect of cardioversion of atrial fibrillation on improvement in left ventricular performance. Am J Cardiol. 2001;88(4):439-441.
doi pubmed - Gillmann HJ, Busche P, Leffler A, Stueber T. Effectiveness of amiodarone versus digitalis for heart rate control in critically ill patients with new-onset atrial fibrillation. Sci Rep. 2022;12(1):2712.
doi pubmed - Kanji S, Williamson DR, Yaghchi BM, Albert M, McIntyre L, Canadian Critical Care Trials G. Epidemiology and management of atrial fibrillation in medical and noncardiac surgical adult intensive care unit patients. J Crit Care. 2012;27(3):326.e321-328.
doi pubmed - O'Bryan LJ, Redfern OC, Bedford J, Petrinic T, Young JD, Watkinson PJ. Managing new-onset atrial fibrillation in critically ill patients: a systematic narrative review. BMJ Open. 2020;10(3):e034774.
doi pubmed - Brown M, Nassoiy S, Chaney W, Plackett TP, Blackwell RH, Luchette F, Engoren M, et al. Impact and treatment success of new-onset atrial fibrillation with rapid ventricular rate development in the surgical intensive care unit. J Surg Res. 2018;229:66-75.
doi pubmed - Yarlagadda B, Vuddanda V, Dar T, Jazayeri MA, Parikh V, Turagam MK, Lavu M, et al. Safety and efficacy of inpatient initiation of dofetilide versus sotalol for atrial fibrillation. J Atr Fibrillation. 2017;10(4):1805.
doi pubmed - Somberg JC, Preston RA, Ranade V, Molnar J. QT prolongation and serum sotalol concentration are highly correlated following intravenous and oral sotalol. Cardiology. 2010;116(3):219-225.
doi pubmed - Nogami A, Kurita T, Abe H, Ando K, Ishikawa T, Imai K, Usui A, et al. JCS/JHRS 2019 guideline on non-pharmacotherapy of cardiac arrhythmias. Circ J. 2021;85(7):1104-1244.
doi pubmed - Zusman O, Amit G, Gilutz H, Zahger D. The significance of new onset atrial fibrillation complicating acute myocardial infarction. Clin Res Cardiol. 2012;101(1):17-22.
doi pubmed - Asanin MR, Vasiljevic ZM, Matic MD, Mrdovic IB, Perunicic JP, Matic DP, Vujisic-Tesic BD, et al. The long-term risk of stroke in patients with acute myocardial infarction complicated with new-onset atrial fibrillation. Clin Cardiol. 2009;32(8):467-470.
doi pubmed - Koniari I, Artopoulou E, Velissaris D, Ainslie M, Mplani V, Karavasili G, Kounis N, et al. Biomarkers in the clinical management of patients with atrial fibrillation and heart failure. J Geriatr Cardiol. 2021;18(11):908-951.
doi pubmed - Mollura M, Lehman LH, Mark RG, Barbieri R. A novel artificial intelligence based intensive care unit monitoring system: using physiological waveforms to identify sepsis. Philos Trans A Math Phys Eng Sci. 2021;379(2212):20200252.
doi pubmed - Varpula M, Tallgren M, Saukkonen K, Voipio-Pulkki LM, Pettila V. Hemodynamic variables related to outcome in septic shock. Intensive Care Med. 2005;31(8):1066-1071.
doi pubmed - Yoshida T, Uchino S, Sasabuchi Y, AFTER-ICU study group. Clinical course after identification of new-onset atrial fibrillation in critically ill patients: The AFTER-ICU study. J Crit Care. 2020;59:136-142.
doi pubmed - January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Jr., Ellinor PT, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104-132.
doi pubmed - Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833.
doi pubmed - Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362(15):1363-1373.
doi pubmed - Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962.
doi pubmed - January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr., Conti JB, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071-2104.
doi pubmed - Kanji S, Stewart R, Fergusson DA, McIntyre L, Turgeon AF, Hebert PC. Treatment of new-onset atrial fibrillation in noncardiac intensive care unit patients: a systematic review of randomized controlled trials. Crit Care Med. 2008;36(5):1620-1624.
doi pubmed - Walkey AJ, Hogarth DK, Lip GYH. Optimizing atrial fibrillation management: from ICU and beyond. Chest. 2015;148(4):859-864.
doi pubmed - Gillinov AM, Bagiella E, Moskowitz AJ, Raiten JM, Groh MA, Bowdish ME, Ailawadi G, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med. 2016;374(20):1911-1921.
doi pubmed - Boriani G, Fauchier L, Aguinaga L, Beattie JM, Blomstrom Lundqvist C, Cohen A, Dan GA, et al. European Heart Rhythm Association (EHRA) consensus document on management of arrhythmias and cardiac electronic devices in the critically ill and post-surgery patient, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin American Heart Rhythm Society (LAHRS). Europace. 2019;21(1):7-8.
doi pubmed - Arrigo M, Jaeger N, Seifert B, Spahn DR, Bettex D, Rudiger A. Disappointing success of electrical cardioversion for new-onset atrial fibrillation in cardiosurgical ICU patients. Crit Care Med. 2015;43(11):2354-2359.
doi pubmed - Sticherling C, Behrens S, Kamke W, Stahn A, Zabel M. Comparison of acute and long-term effects of single-dose amiodarone and verapamil for the treatment of immediate recurrences of atrial fibrillation after transthoracic cardioversion. Europace. 2005;7(6):546-553.
doi pubmed - Shima N, Miyamoto K, Kato S, Yoshida T, Uchino S, AFTER-ICU study group. Primary success of electrical cardioversion for new-onset atrial fibrillation and its association with clinical course in non-cardiac critically ill patients: sub-analysis of a multicenter observational study. J Intensive Care. 2021;9(1):46.
doi pubmed - Saylik F, Cinar T, Akbulut T, Hayiroglu MI. Comparison of catheter ablation and medical therapy for atrial fibrillation in heart failure patients: A meta-analysis of randomized controlled trials. Heart Lung. 2023;57:69-74.
doi pubmed - Lavee J, Onik G, Mikus P, Rubinsky B. A novel nonthermal energy source for surgical epicardial atrial ablation: irreversible electroporation. Heart Surg Forum. 2007;10(2):E162-167.
doi pubmed - Darwish OS, Strube S, Nguyen HM, Tanios MA. Challenges of anticoagulation for atrial fibrillation in patients with severe sepsis. Ann Pharmacother. 2013;47(10):1266-1271.
doi pubmed - Walkey AJ, Quinn EK, Winter MR, McManus DD, Benjamin EJ. Practice patterns and outcomes associated with use of anticoagulation among patients with atrial fibrillation during sepsis. JAMA Cardiol. 2016;1(6):682-690.
doi pubmed - Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719-2747.
doi pubmed - Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126(7):860-865.
doi pubmed - Semeraro N, Ammollo CT, Semeraro F, Colucci M. Coagulopathy of acute sepsis. Semin Thromb Hemost. 2015;41(6):650-658.
doi pubmed - Umemura Y, Yamakawa K, Ogura H, Yuhara H, Fujimi S. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost. 2016;14(3):518-530.
doi pubmed - Yamakawa K, Umemura Y, Hayakawa M, Kudo D, Sanui M, Takahashi H, Yoshikawa Y, et al. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit Care. 2016;20(1):229.
doi pubmed - Keller M, Meierhenrich R. [New onset atrial fibrillation in patients with sepsis]. Anaesthesist. 2017;66(10):786-794.
doi pubmed - Zarei B, Bozorgi A, Khoshfetrat M, Arefizadeh R, Mohsenizadeh SA, Mousavi SH, Jalali A, et al. Incidence and predictors of new-onset atrial fibrillation in ST-elevation myocardial infarction: A single-center study. Health Sci Rep. 2024;7(7):e2226.
doi pubmed - Schnabel RB, Marinelli EA, Arbelo E, Boriani G, Boveda S, Buckley CM, Camm AJ, et al. Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. Europace. 2023;25(1):6-27.
doi pubmed - Dai H, Ye J, Wang S, Li X, Li W. Myeloperoxidase and its derivative hypochlorous acid combined clinical indicators predict new-onset atrial fibrillation in sepsis: a case-control study. BMC Cardiovasc Disord. 2024;24(1):377.
doi pubmed - Beyls C, Hermida A, Daumin C, Delmotte MP, Nsiku A, Huette P, Bunelle C, et al. Left and right atrial strain analysis to predicting new-onset atrial fibrillation in patients with septic shock: a single-center retrospective echocardiography study. Crit Care. 2024;28(1):233.
doi pubmed - Bashar SK, Hossain MB, Ding E, Walkey AJ, McManus DD, Chon KH. Atrial fibrillation detection during sepsis: study on MIMIC III ICU data. IEEE J Biomed Health Inform. 2020;24(11):3124-3135.
doi pubmed - Walkey AJ, Bashar SK, Hossain MB, Ding E, Albuquerque D, Winter M, Chon KH, et al. Development and validation of an automated algorithm to detect atrial fibrillation within stored intensive care unit continuous electrocardiographic data: observational study. JMIR Cardio. 2021;5(1):e18840.
doi pubmed - Odening KE, Deiss S, Dilling-Boer D, Didenko M, Eriksson U, Nedios S, Ng FS, et al. Mechanisms of sex differences in atrial fibrillation: role of hormones and differences in electrophysiology, structure, function, and remodelling. Europace. 2019;21(3):366-376.
doi pubmed - Salih M, Abdel-Hafez O, Ibrahim R, Nair R. Atrial fibrillation in the elderly population: Challenges and management considerations. J Arrhythm. 2021;37(4):912-921.
doi pubmed - Chen N, Alam AB, Lutsey PL, MacLehose RF, Claxton JS, Chen LY, Chamberlain AM, et al. Polypharmacy, adverse outcomes, and treatment effectiveness in patients >/=75 with atrial fibrillation. J Am Heart Assoc. 2020;9(11):e015089.
doi pubmed - Shaikh F, Pasch LB, Newton PJ, Bajorek BV, Ferguson C. Addressing multimorbidity and polypharmacy in individuals with atrial fibrillation. Curr Cardiol Rep. 2018;20(5):32.
doi pubmed - Lin Z, Han H, Guo W, Wei X, Guo Z, Zhai S, Li S, et al. Atrial fibrillation in critically ill patients who received prolonged mechanical ventilation: a nationwide inpatient report. Korean J Intern Med. 2021;36(6):1389-1401.
doi pubmed - Azahar SN, Sulong S, Wan Zaidi WA, Muhammad N, Kamisah Y, Masbah N. Direct medical cost of stroke and the cost-effectiveness of direct oral anticoagulants in atrial fibrillation-related stroke: a cross-sectional study. Int J Environ Res Public Health. 2022;19(3):1078.
doi pubmed - Steinberg BA, Dorian P, Anstrom KJ, Hess R, Mark DB, Noseworthy PA, Spertus JA, et al. Patient-reported outcomes in atrial fibrillation research: results of a clinicaltrials.gov analysis. JACC Clin Electrophysiol. 2019;5(5):599-605.
doi pubmed - Steinberg BA, Turner J, Lyons A, Biber J, Chelu MG, Fang JC, Freedman RA, et al. Systematic collection of patient-reported outcomes in atrial fibrillation: feasibility and initial results of the Utah mEVAL AF programme. Europace. 2020;22(3):368-374.
doi pubmed - Ali-Ahmed F, Pieper K, North R, Allen LA, Chan PS, Ezekowitz MD, Fonarow GC, et al. Shared decision-making in atrial fibrillation: patient-reported involvement in treatment decisions. Eur Heart J Qual Care Clin Outcomes. 2020;6(4):263-272.
doi pubmed - Johnston B, Hill RA, Blackwood B, Lip GYH, Welters ID. Development of core outcome sets for trials on the management of atrial fibrillation in critically unwell patients (COS-ABACUS): a protocol. BMJ Open. 2023;13(4):e067257.
doi pubmed - Arunachalam K, Kalyan Sundaram A, Jha K, Thakur L, Pond K. Evaluation of anticoagulation practice with new-onset atrial fibrillation in patients with sepsis and septic shock in medical intensive care unit: a retrospective observational cohort study. Cureus. 2020;12(8):e10026.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.