| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Case Report
Volume 16, Number 1, February 2025, pages 72-79
First Transcatheter Valve-in-Valve Implantation With Myval Octacor Into a Failed Biological Prosthetic Aortic Valve in Serbia
Valentina Balint Jovanovica, Mihajlo Farkica, e, Darko Boljevica, Milovan Bojica, b, Matija Furtulaa, Dragan Topica, Milan Dobrica, c, Alfonso Ielasid, Vladimir Zobenicaa, Ida Subotica, Aleksandra Nikolica, c
aDedinje Cardiovascular Institute, Belgrade, Serbia
bSchool of Medicine, University of Banja Luka, Banja Luka, Bosnia and Herzegovina
cSchool of Medicine, University of Belgrade, Belgrade, Serbia
dU.O. Cardiologia Ospedaliera, IRCCS Ospedale Galeazzi-Sant-Ambrogio, Milan, Italy
eCorresponding Author: Mihajlo Farkic, Dedinje Cardiovascular Institute, Belgrade 11000, Serbia
Manuscript submitted November 4, 2024, accepted November 13, 2024, published online January 4, 2025
Short title: First ViV-TAVR With Myval Octacor in Serbia
doi: https://doi.org/10.14740/cr1751
| Abstract | ▴Top |
The natural progression of bioprosthetic valve degeneration over time requires further interventions for those experiencing symptomatic prosthesis dysfunction. Transcatheter aortic valve replacement (TAVR) emerges as a promising therapeutic option to alleviate symptoms in such patients. The valve-in-valve (ViV) technique eliminates the necessity for repetitive open-heart surgical procedures, offering particular advantages for individuals with higher surgical risks. In this report, we describe the case of a 78-year-old female patient presenting with severe symptomatic aortic restenosis of a biological aortic valve implanted 5 years prior. Given the patient’s high surgical risk, a transcatheter ViV implantation was chosen as the treatment approach. Utilizing a balloon-expandable valve, the intervention resulted in the successful implantation of a functional TAVR, resulting in symptom relief and enabling a fast discharge from the hospital.
Keywords: Aortic restenosis; Balloon-expandable valve; Failed surgical bioprostheses; Myval Octacor; Transcatheter aortic valve replacement; Transcatheter heart valve; Valve-in-valve
| Introduction | ▴Top |
The long-term survival rates in patients with surgical aortic bioprostheses are high. Nevertheless, bioprosthetic valves have a limited longevity of 10 - 20 years, and valve deterioration is frequently observed. The natural progression of bioprosthetic valve degeneration over time requires further interventions for those experiencing symptomatic prosthesis dysfunction. Therefore, it is estimated that the number of patients requiring re-treatment for surgical aortic bioprostheses failure is likely to rise within the next years [1-3].
Transcatheter aortic valve replacement (TAVR) emerges as a promising treatment to alleviate symptoms in such patients, bypassing the need for prolonged post-surgical recovery. The valve-in-valve (ViV) technique eliminates the necessity for repetitive open-heart surgical procedures, offering particular advantages for individuals with higher surgical risks attributed to factors such as advanced age, comorbidities, and the likelihood of recurrent cardiac surgical interventions [1-4].
Although the outcomes of ViV-TAVR are generally satisfactory, it is essential to note that the procedure still carries a 30-day mortality rate of 7.6% and a 1-year survival rate of 83.2% [5]. This case report aims to describe the first ViV-TAVR procedure using a new-generation balloon-expandable (BE) Myval Octacor transcatheter heart valve (THV) in a failed biological prosthetic aortic valve of a high-risk patient in Serbia. We present the procedural approach, valve selection, and outcomes up to a 6-month follow-up to evaluate the feasibility, safety, and efficacy of the Myval Octacor THV in this complex, high-risk ViV scenario. This report provides insights into the application of next-generation THV technologies in challenging patient populations.
| Case Report | ▴Top |
Investigations
Here, we present the case of a 78-year-old female patient exhibiting severe symptomatic aortic restenosis of her biological aortic valve, which was initially implanted 5 years ago.
Over the preceding year, the patient has experienced dyspnea upon mild exertion and intermittent chest pain during physical activity. There have been no episodes of syncope; however, the patient has reported frequent falls attributed to dizziness, resulting in multiple, minor skeletal traumas. Following the decision of the heart team to forego surgical intervention due to high surgical risk, we decided on a transcatheter ViV implantation as the treatment strategy. Employing a BE TAVR, we successfully implanted it within the ring of the failed biological valve. The procedure was conducted under cerebral protection. Subsequently, the intervention resulted in a functional TAVR, alleviation of symptoms, and facilitated early discharge from the hospital. The patient presented with a body mass index (BMI) of 34.7 kg/m2, afebrile, a pulse rate of 60 beats per minute, and a blood pressure of 120/70 mm Hg. Auscultation revealed an ejection systolic murmur, most pronounced over the aortic valve. Additionally, lower extremity pretibial edema was noted. Limited movement in the right shoulder was attributed to prior trauma. No other significant abnormalities were detected during the systematic physical examination.
Diagnosis
The laboratory findings showed white blood cell (WBC) of 6.3 × 109/L, hemoglobin (Hb) of 107 g/L, platelet count of 275 × 109/L, creatinine of 98 µmol/L, urea of 9.81 mmol/L, and N-terminal pro B-type natriuretic peptide (NTproBNP) of 980 pg/mL. Risk scores were calculated: Society of Thoracic Surgeons score for operative mortality was 4.12%, overall morbidity and mortality score was 11.6%, and EuroScore II was 6.54%. Clinical frailty score was 5 at the time of admission.
The electrocardiogram (ECG) showed sinus rhythm, alongside a chronic left bundle branch block, characterized by a QRS duration of 130 ms. On transthoracic echocardiography (TTE), an artificial biological aortic valve was identified in the aortic position. A mean gradient of 39.60 mm Hg and a maximal gradient of 69.81 mm Hg were measured. Trivial transvalvular aortic regurgitation was noted, and left ventricular ejection fraction (LVEF) was preserved at 55% (Fig. 1).
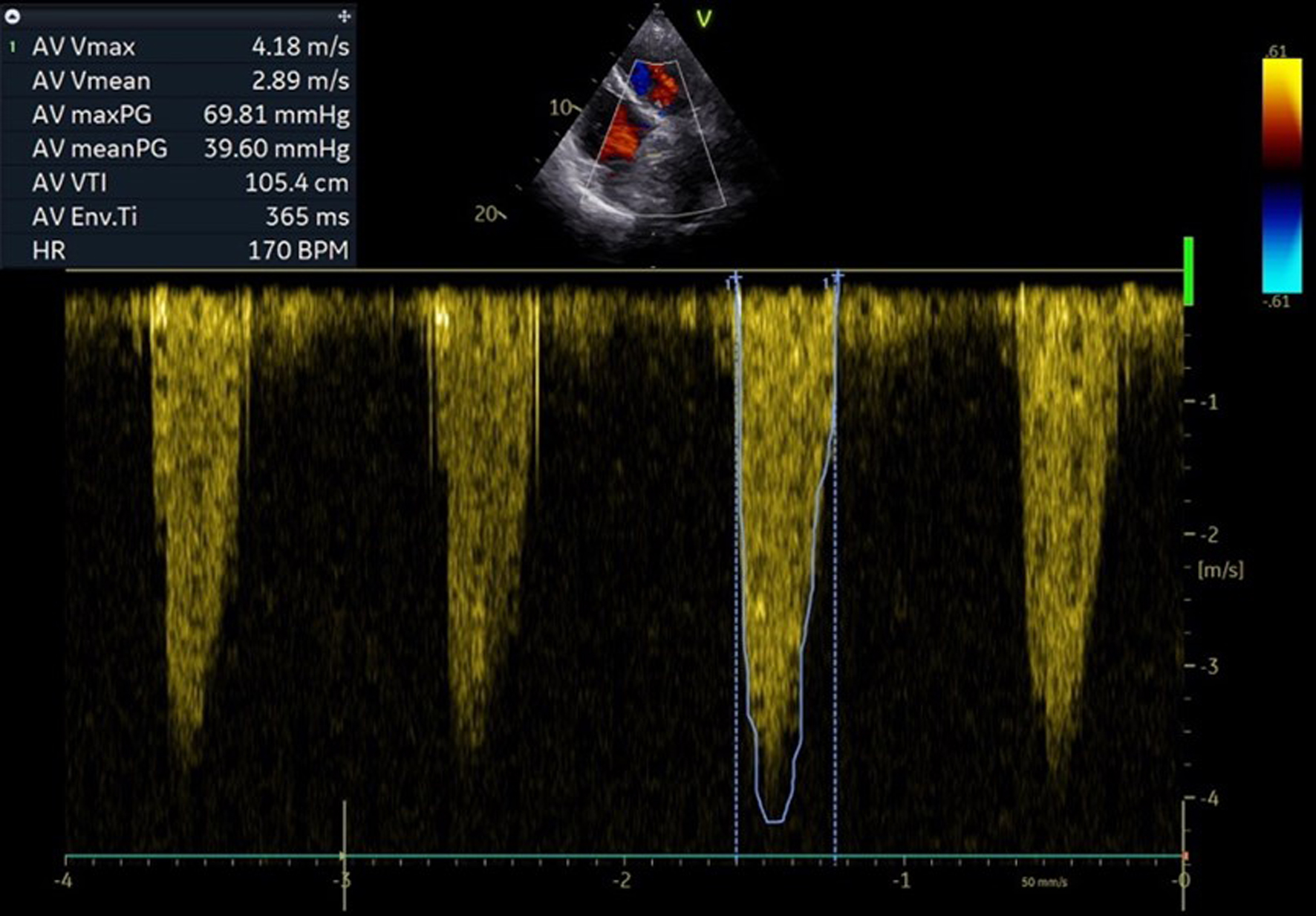 Click for large image | Figure 1. Pre-procedural echocardiographic pressure gradients over surgical aortic bioprostheses. |
Coronary angiography revealed no hemodynamically significant stenosis. Additionally, color Doppler imaging of the carotid arteries indicated the absence of significant atherosclerotic lesions. Infective endocarditis was ruled out as the underlying cause of valve degeneration.
A computed tomography (CT) scan of the thoracic and abdominal aorta was performed to assess the aortic root and valve annulus, aiding in the selection of an appropriate valve size and its placement. The scan revealed optimal heights of the left and right coronary ostia, with the right measuring 21 mm and the left 19 mm. The perimeter derived of the annulus, as determined by CT, was 20.8 mm (Fig. 2). Additionally, optimal heights of the sinuses of Valsalva were observed (left coronary cusp ((LCC) 22 mm, right coronary cusp (RCC) 26 mm, non-coronary cusp (NCC) 24.5 mm)) (Fig. 3). The aortic root angulation was measured at 30°, and the CT revealed separate origins of the brachiocephalic trunk, left carotid artery, and left subclavian artery suitable for cerebral protection device (Fig. 4). Based on these measurements, the BE Myval Octacor 23 mm +2 cc valve (Meril Life Sciences Pvt. Ltd, India) was chosen for implantation.
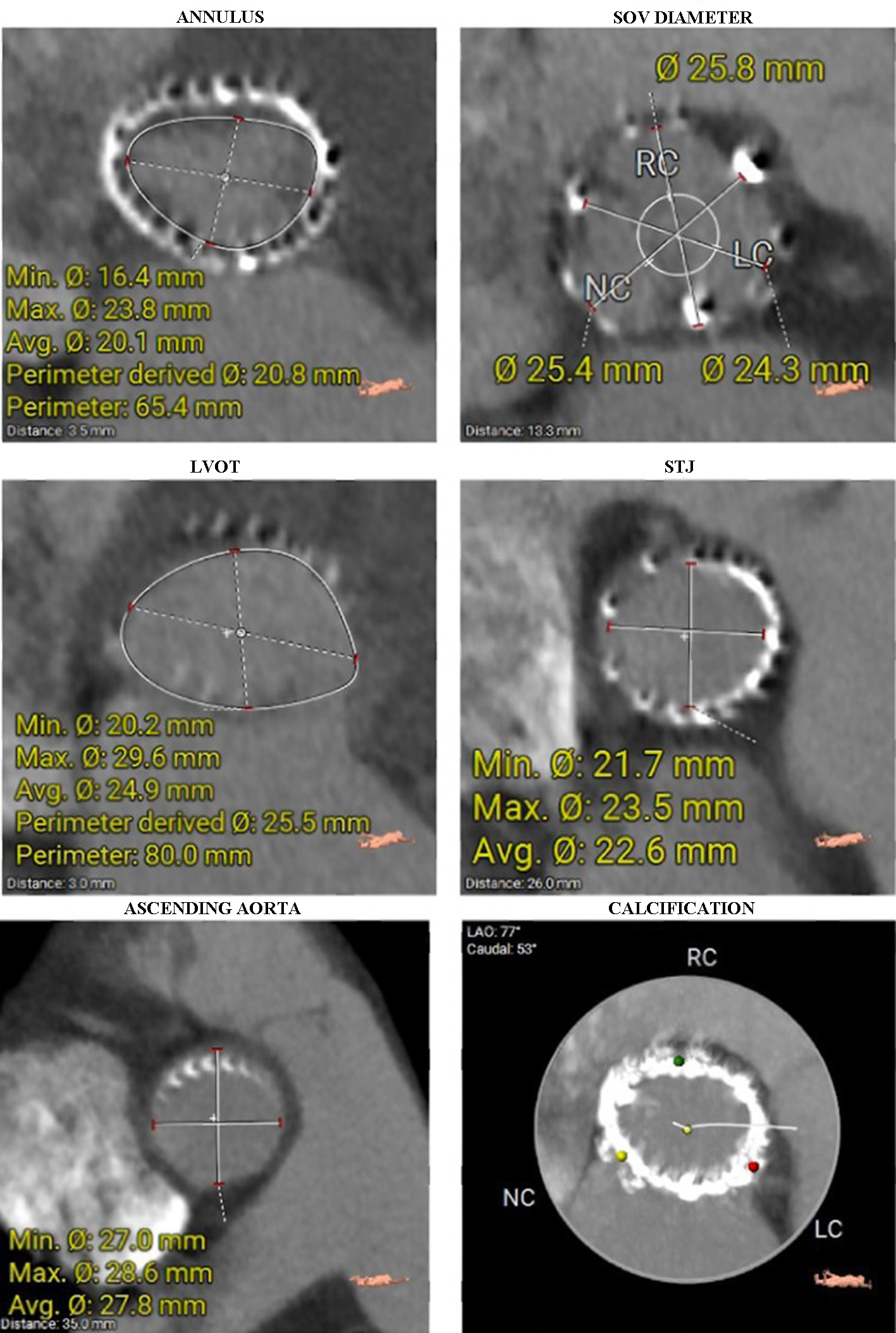 Click for large image | Figure 2. Diameters of the aortic annulus, SOV, LVOT, STJ, and ascending aorta were measured on CT. The last image shows a heavily calcified aortic annulus. CT: computed tomography; LVOT: left ventricular outflow tract; SOV: sinus of Valsalva; STJ: sinotubular junction. |
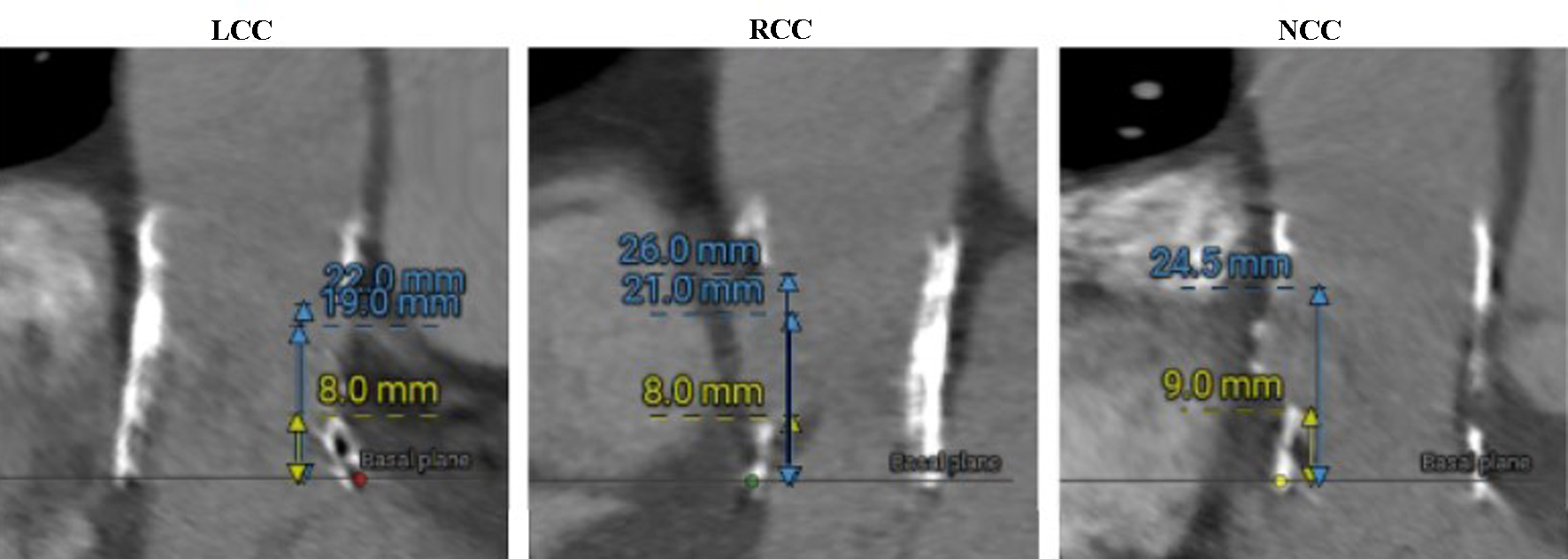 Click for large image | Figure 3. Sinus of Valsalva heights measured on CT (LCC, RCC, and NCC, respectively). CT: computed tomography; LCC: left coronary cusp; NCC: non-coronary cusp; RCC: right coronary cusp. |
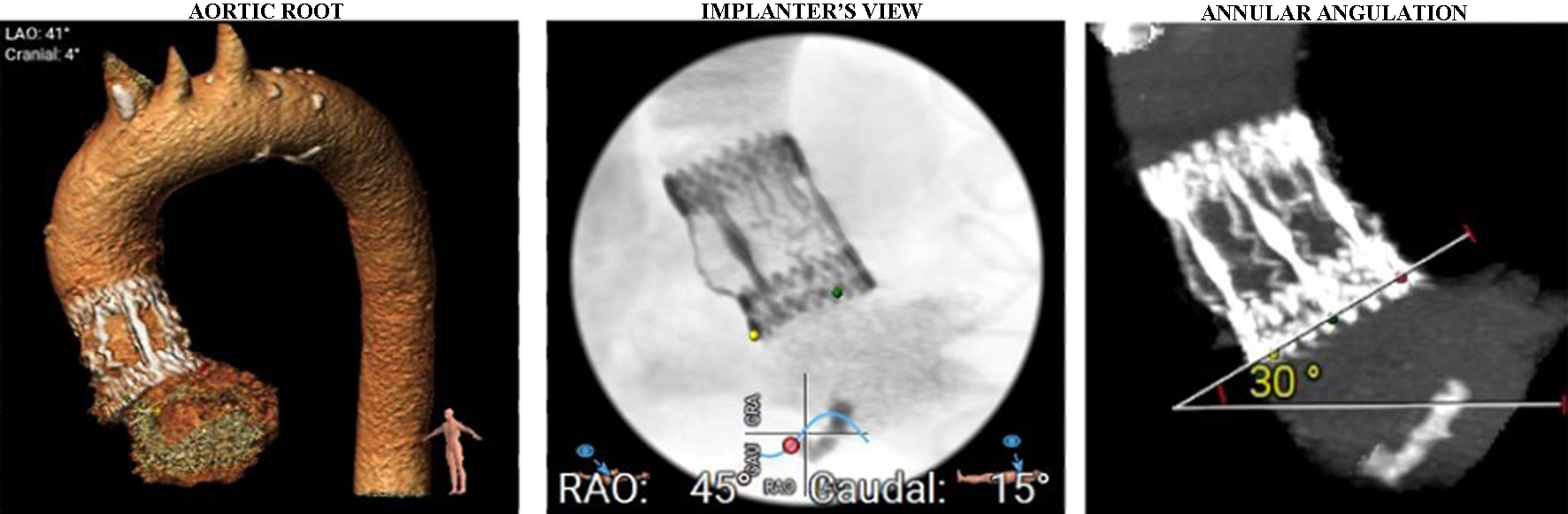 Click for large image | Figure 4. Aortic arch and aortic root angulation. Separate origins of brachiocephalic trunk, left carotid artery, and left subclavian artery were seen. |
No significant tortuosity was seen in the left and right iliac arteries, and the CT scan revealed no notable calcification (Fig. 5). The right femoral artery was selected as the primary access route.
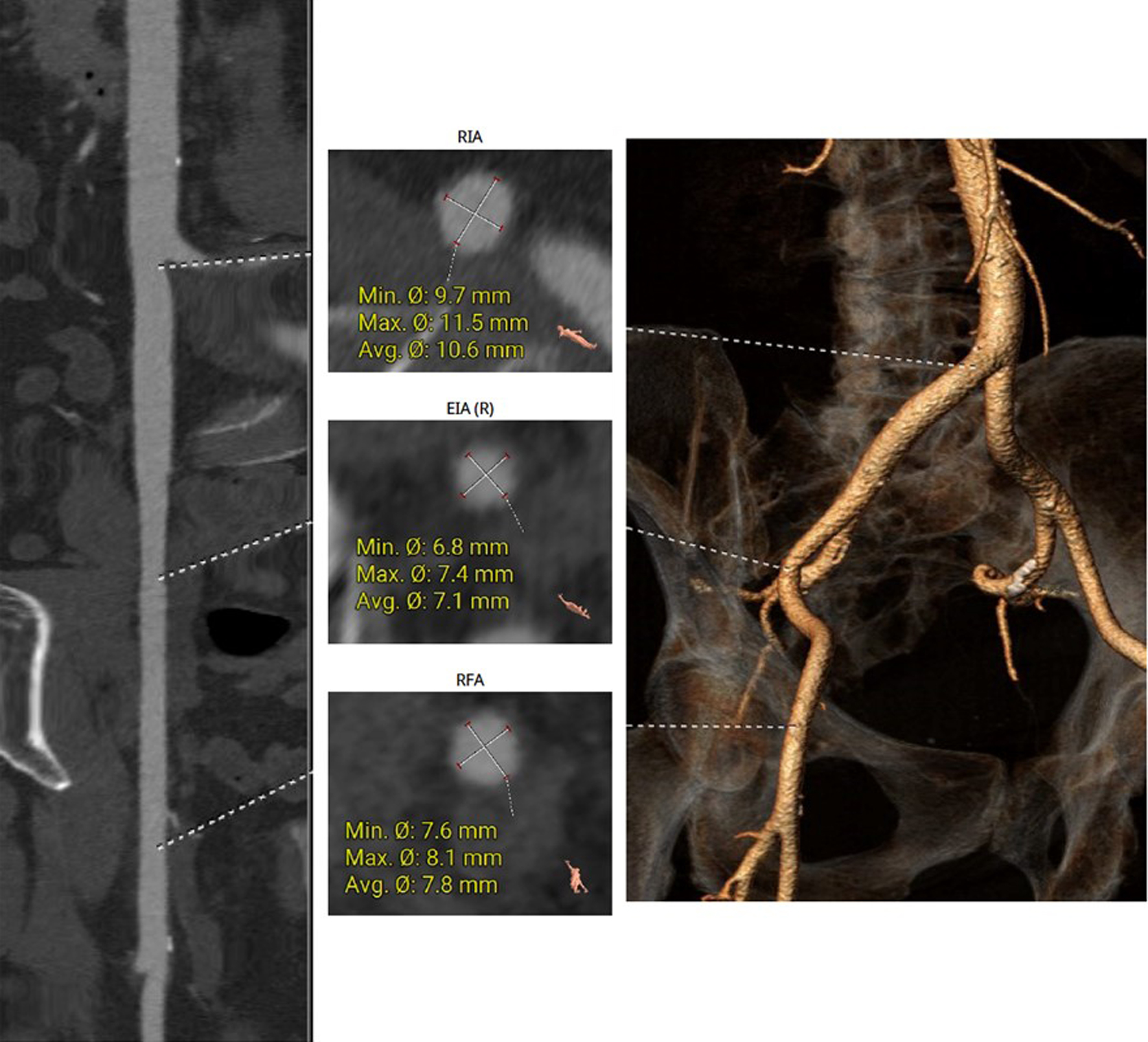 Click for large image | Figure 5. The right femoral artery was chosen for the main access site, without significant tortuosity or notable calcification seen on CT. CT: computed tomography. |
Treatment
Based on the pre-procedural diagnostic assessment, the cardiac team opted for the use of a BE Myval Octacor 23 mm +2 cc valve (Meril Life Sciences Pvt. Ltd, Gujarat, India). This selection was made based on its appropriate sizing, radial force, and shorter stent frame height. Careful planning was undertaken to consider the anatomical aspects of the previously implanted biological aortic valve (Sorin Perceval L (Sorin, Salluggia, Italy)). A bifemoral approach was obtained by positioning an 18-F transcatheter aortic valve introducer in the right femoral artery and a 6-F introducer for a pigtail catheter in the left femoral artery. Safari small wire was inserted into the left ventricle, while cerebral protection was provided via the right radial artery using the Sentinel device. Direct implantation of Myval Octacor 23 mm +2 cc was performed. Post-dilation with the +1 cc balloon was conducted due to residual pressure gradient over the valve (Fig. 6). Control angiography and echocardiographic assessment demonstrated optimal alignment of the prosthetic valve with no residual aortic regurgitation. During the procedure, a transient atrioventricular block occurred, so a pacemaker electrode was retained as a precautionary measure. However, no significant conduction abnormalities were detected on Holter monitor ECG during in-hospital stay, and therefore, there was no indication for a permanent pacemaker implantation (PPI).
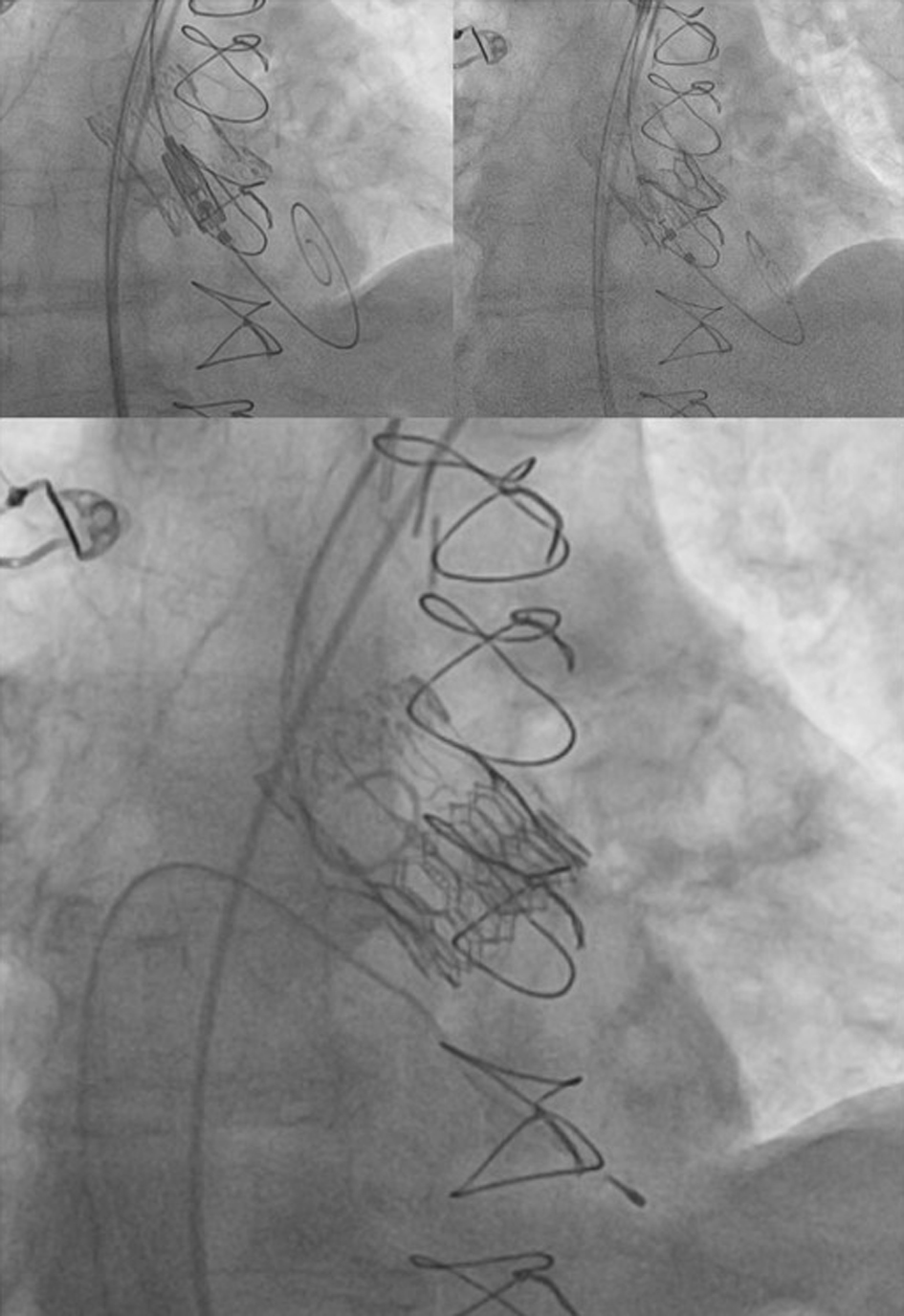 Click for large image | Figure 6. Valve-in-valve TAVR - implantation of ballon-expandable Myval Octacor valve into previously implanted bioprothesis. TAVR: transcatheter aortic valve replacement. |
Follow-up and outcomes
Post-procedural echocardiography showed optimal results with the transcatheter aortic valve which expanded well. A mean gradient of 15.37 mm Hg and a maximal gradient of 30.83 mm Hg were measured, with trivial aortic regurgitation (Fig. 7).
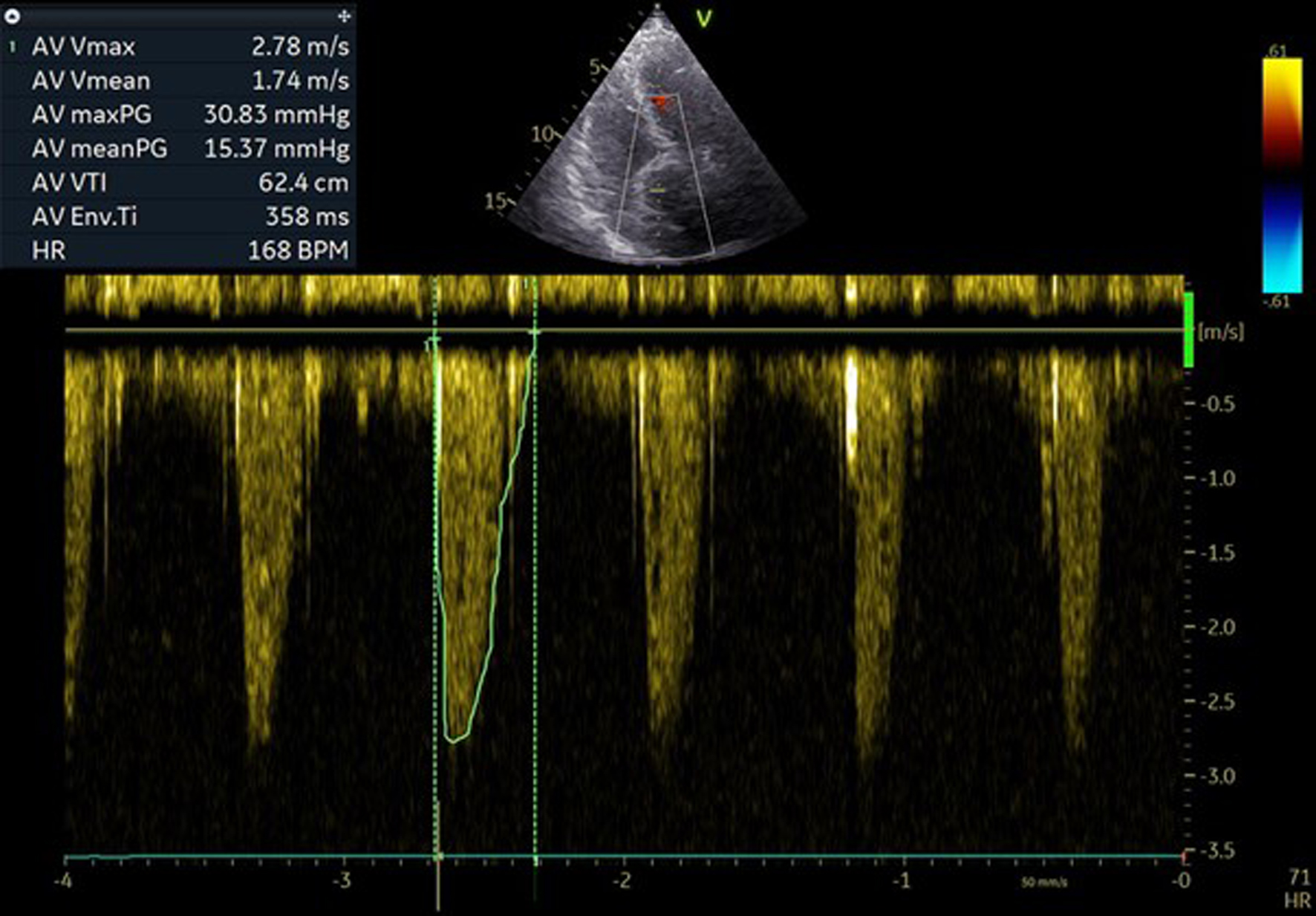 Click for large image | Figure 7. Post-procedural pressure gradients over aortic valve. |
At 1-month follow-up, the patient exhibited residual exertional dyspnea classified as New York Heart Association class II, with symptomatic improvement after increase in diuretic therapy. The patient reported no episodes of dizziness, nor chest pain. However, a decline in Hb levels to 81 g/L prompted evaluation by a gastroenterologist. A follow-up echocardiography revealed a mean gradient of 11 mm Hg and a maximal gradient of 22 mm Hg, with trivial aortic regurgitation.
At the 6-month follow-up, the patient’s exertional dyspnea had worsened, and a further increase in diuretic therapy was made. However, Hb levels had improved to 129 g/L following iron supplementation. Echocardiographic assessment demonstrated a mean gradient of 21 mm Hg, a maximal gradient of 36 mmHg, and trivial aortic regurgitation. Additionally, moderate tricuspid regurgitation with a systolic pulmonary artery pressure of 50 mm Hg was measured.
| Discussion | ▴Top |
This is the first case report with BE Myval Octacor implanted in a failed surgical valve bioprostheses. In the current case, the patient’s advanced age, history of prior open-heart surgery, and relatively high frailty score led us to opt for a less invasive approach. The choice of the valve was influenced by the valve’s strong radial force, aiming to potentially expand the underlying frame of the previously implanted biological valve. Additionally, the low skirt height reduces the likelihood of coronary artery occlusion. The mean gradient measured approximately 20 mm Hg during the 6-month follow-up period. There was no post-procedural aortic regurgitation. The patient reported a notable absence of dizziness and chest pain, symptoms which were frequently experienced prior to the procedure. However, her tolerance for physical activity remained suboptimal, potentially attributable to anemia and elevated right heart filling pressure.
As ViV procedures are challenging, few studies have discussed the outcomes and challenges of these procedures. A matched analysis conducted by Tuzcu et al revealed lower 30-day mortality (2.9% vs. 4.8%; P < 0.001) in the ViV-TAVR compared with the native-TAVR group. Comparison with the benchmark native-TAVR shows ViV-TAVR to be a safe and effective procedure in patients with failed SAVR who are at high risk for repeat surgery [2]. According to the VIVID registry, clinical outcomes following aortic ViV-TAVR are significantly influenced by the characteristics of the existing surgical valve. Specifically, smaller and stenotic surgical valves are linked to poorer clinical outcomes. The results from the VIVID registry reveal that patients with small surgical valves (≤ 21 mm) experience worse 1-year mortality rates after ViV-TAVR compared to those with intermediate and large surgical valves. The 1-year mortality rates were 25.2% for patients with small valves, compared to 18.2% for those with intermediate-sized valves and 6.7% for those with large valves [4, 5].
Recent research has investigated the application of both BE and self-expandable (SE) valves in TAVR for failed bioprosthetic valves. Deeb and colleagues found that the use of SE valves (CoreValve bioprosthesis (Medtronic, Minneapolis, Minnesota)) in patients who underwent TAVR with failed surgical bioprosthesis resulted in acceptable hemodynamics and low rates of moderate residual aortic regurgitation at 1 and 12 months post-procedure. Moreover, higher residual aortic valve gradients at 1 month were associated with smaller surgical valve size, stenosis as the cause of failure, and the degree of patient-prosthesis mismatch (PPM) [6]. Another trial VIVA included two SE valves (CoreValve or Evolut R (Medtronic, Minneapolis, Minnesota)), and they confirmed the safety and efficacy of the procedure. The trial demonstrated low 1-year mortality rates (8.8% all-cause, 5.6% cardiovascular) and relatively low occurrence of clinical endpoints such as stroke, acute kidney injury, and new PPI, all of which remained within acceptable ranges. Notably, this trial’s distinctiveness lies in its extensive utilization of small-inner diameter surgical valves [7].
The ViV-TAVR study included both SE and BE valves, and primary findings revealed that ViV-TAVR effectively reduces transvalvular gradient and resolves aortic regurgitation. However, residual stenosis (mean pressure gradient > 20 mm Hg) is common, particularly in patients with small surgical bioprosthetic valve sizes, though it does not increase mortality rates [8]. These findings are consistent with Bleiziffer et al’s study but differ from earlier reports on surgical valve replacement, where residual stenosis was often associated with PPM [9-11]. Hence, the results from these trials have consistently affirmed the safety and efficacy of ViV-TAVR for failed surgical aortic valve bioprosthesis with satisfactory outcomes, including low 1-year mortality rates and a low incidence of complications like stroke, acute kidney injury, and the requirement for new PPI.
Conclusion
This case highlights the feasibility and safety of the successful implantation of a BE Myval Octacor transcatheter heart valve into a non-functional biological aortic valve in a high-risk patient. This alternative therapeutic approach could be considered for patients who are unsuitable candidates for redo surgical interventions.
Learning points
This is the first case report of BE Myval Octacor THV implanted in a failed surgical bioprosthetic valve. This case highlights the effective use of a less invasive treatment approach for patients with advanced age, a history of prior open-heart surgery, and a relatively high frailty score. The Myval Octacor THV was selected due to its strong radial force, aiming to potentially expand the underlying frame of the previously implanted biological valve. There were no procedural complications or mortality during 6-month follow-up period. This case underscores the importance of considering TAVR as a viable alternative to traditional open-heart surgery in managing valve degeneration and improving patient outcomes.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient to publish the data.
Author Contributions
Study design: MF, DB and AI; investigation: all authors; data collection: all authors; writing - original draft: all authors; writing - review and editing: all authors; visualization: all authors; supervision: MF and AI.
Data Availability
The authors declare that the data supporting the findings of this study are available within the article.
Abbreviations
BE: balloon-expandable; BMI: body mass index; CT: computed tomography; ECG: electrocardiogram; Hb: hemoglobin; LCC: left coronary cusp; LVEF: left ventricular ejection fraction; NCC: non-coronary cusp; PPI: permanent pacemaker implantation; PPM: patient-prosthesis mismatch; RCC: right coronary cusp; SE: self-expandable; TAVR: transcatheter aortic valve replacement; THV: transcatheter heart valve; TTE: transthoracic echocardiography; ViV: valve-in-valve; WBC: white blood cell
| References | ▴Top |
- Dvir D, Bourguignon T, Otto CM, Hahn RT, Rosenhek R, Webb JG, Treede H, et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. 2018;137(4):388-399.
doi pubmed - Tuzcu EM, Kapadia SR, Vemulapalli S, Carroll JD, Holmes DR, Jr., Mack MJ, Thourani VH, et al. Transcatheter aortic valve replacement of failed surgically implanted bioprostheses: the STS/ACC registry. J Am Coll Cardiol. 2018;72(4):370-382.
doi pubmed - Fukunaga N, Okada Y, Konishi Y, Murashita T, Yuzaki M, Shomura Y, Fujiwara H, et al. Clinical outcomes of redo valvular operations: a 20-year experience. Ann Thorac Surg. 2012;94(6):2011-2016.
doi pubmed - Dvir D, Webb J, Brecker S, Bleiziffer S, Hildick-Smith D, Colombo A, Descoutures F, et al. Transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: results from the global valve-in-valve registry. Circulation. 2012;126(19):2335-2344.
doi pubmed - Dvir D, Webb JG, Bleiziffer S, Pasic M, Waksman R, Kodali S, Barbanti M, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312(2):162-170.
doi pubmed - Deeb GM, Chetcuti SJ, Reardon MJ, Patel HJ, Grossman PM, Schreiber T, Forrest JK, et al. 1-year results in patients undergoing transcatheter aortic valve replacement with failed surgical bioprostheses. JACC Cardiovasc Interv. 2017;10(10):1034-1044.
doi pubmed - Tchetche D, Chevalier B, Holzhey D, Harnath A, Schafer U, Teiger E, Manigold T, et al. TAVR for failed surgical aortic bioprostheses using a self-expanding device: 1-year results from the prospective VIVA postmarket study. JACC Cardiovasc Interv. 2019;12(10):923-932.
doi pubmed - Wernly B, Zappe AK, Unbehaun A, Sinning JM, Jung C, Kim WK, Fichtlscherer S, et al. Transcatheter valve-in-valve implantation (VinV-TAVR) for failed surgical aortic bioprosthetic valves. Clin Res Cardiol. 2019;108(1):83-92.
doi pubmed - Bleiziffer S, Eichinger WB, Hettich I, Ruzicka D, Wottke M, Bauernschmitt R, Lange R. Impact of patient-prosthesis mismatch on exercise capacity in patients after bioprosthetic aortic valve replacement. Heart. 2008;94(5):637-641.
doi pubmed - Urso S, Sadaba R, Vives M, Trujillo J, Beltrame S, Soriano B, Piqueras L, et al. Patient-prosthesis mismatch in elderly patients undergoing aortic valve replacement: impact on quality of life and survival. J Heart Valve Dis. 2009;18(3):248-255.
pubmed - Pibarot P, Weissman NJ, Stewart WJ, Hahn RT, Lindman BR, McAndrew T, Kodali SK, et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: a PARTNER trial cohort—a analysis. J Am Coll Cardiol. 2014;64(13):1323-1334.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.