| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 2, April 2025, pages 102-109
Long-Term Clinical Outcomes in Patients With Transthyretin Cardiac Amyloidosis Versus Non-Ischemic Cardiomyopathy
Barbara C. Okekea, Timothy Chruscielb, Mina M. Benjaminc, d
aDepartment of Internal Medicine, St. Louis University Hospital, St. Louis, MO, USA
bAdvanced Health Data (AHEAD) Institute, Saint Louis University School of Medicine, St. Louis, MO, USA
cDivision of Cardiology, Department of Internal Medicine, St. Louis University Hospital, St. Louis, MO, USA
dCorresponding Author: Mina M. Benjamin, Division of Cardiology, Department of Internal Medicine, St. Louis University Hospital, St. Louis, MO 63104, USA
Manuscript submitted January 24, 2025, accepted February 13, 2025, published online February 22, 2025
Short title: Outcomes in Transthyretin CA Versus NICM
doi: https://doi.org/10.14740/cr2050
| Abstract | ▴Top |
Background: We sought to compare the long-term outcomes in patients with transthyretin cardiac amyloidosis (CA) compared to those with non-ischemic cardiomyopathy (NICM) from a large healthcare system database.
Methods: Patients with CA or NICM were identified from SSM Healthcare System’s data warehouse using ICD codes. Inclusion criteria included at least 6 months of follow-up. Outcomes studied were heart failure hospitalization (HFH), ventricular tachyarrhythmias (VTA), implantable cardiac defibrillator (ICD) and pacemaker (PM) placement. Multivariate logistic analysis and Kaplan-Meier survival curves were constructed.
Results: We identified 231 patients with CA and 462 with NICM, matched for age, race, and gender. CA patients had higher incidence of peripheral vascular disease (48.5% vs. 35.5%) and coronary artery disease (10.4% vs. 6.1%). Mean follow-up was 48.1 ± 33.1 months. CA patients had a higher rate of HFH (57.6% vs. 46.1%) and a lower rate of ICD (1.7% vs. 5.9%). In the multivariate model, CA patients had significantly higher odds for HFH (odds ratio: 1.86; 95% confidence interval: 1.29 - 2.68). Kaplan-Meier survival curves showed a trend toward earlier HFH and later PM or ICD implantation in CA patients.
Conclusions: In this retrospective study from a large healthcare system database, compared to NICM, transthyretin CA patients had significantly higher rates of HFH, similar odds of VTA, and a lower likelihood of receiving an intracardiac device.
Keywords: Cardiac amyloidosis; Non-ischemic cardiomyopathy; Pacemaker; Transthyretin; Heart failure
| Introduction | ▴Top |
Cardiac amyloidosis (CA) is a form of restrictive cardiomyopathy caused by amyloid fibril deposition in the extracellular space of the heart. Among patients who have CA, approximately 95% of cases are caused by the deposition of transthyretin (ATTR amyloidosis) or immunoglobulin light chains (AL amyloidosis). CA is associated with both systolic and diastolic heart failure (HF), which has significant morbidity and mortality [1] and should be managed differently from other forms of non-ischemic cardiomyopathy (NICM) [2]. While there has been an increase in prevalence over the last 20 years, the incidence of CA has remained relatively stable [3, 4]. NICM is a broad term that includes patients whose cardiomyopathy is not primarily caused by obstructive coronary artery disease. The most common form of NICM is dilated cardiomyopathy with a reduced left ventricular ejection fraction (LVEF). Other complications of CA, other than HF, include atrial arrhythmias, ventricular tachyarrhythmias (VTA) and valvular heart disease [5-7]. We sought to compare the long-term clinical outcomes in patients with transthyretin CA versus NICM patients from a large healthcare system database.
| Materials and Methods | ▴Top |
CA and NICM patients were identified from SSM Healthcare System’s data warehouse using ICD codes. SLU-SSM is a member site of the Health Care Systems Research Network (HCSRN) [8] and the virtual data warehouse (VDW) was created and is maintained per HCSRN specifications. The SSM healthcare system includes locations in Missouri, Illinois, Oklahoma, and Wisconsin. The VDW contains de-identified clinical data for over 5 million patients dating back to 2008. This includes ICD-9 and ICD-10 codes, procedure codes, pharmacy orders, vital signs, laboratory tests, and demographics. The ICD codes included the most recent ICD-10 designation specific for cardiac amyloid, E85.4, and some who had combined ICD-9 codes including 425.7 were also used to isolate patients with CA. Patients with the code 277.39 were excluded to isolate patients with transthyretin CA. Because patients do not actively participate and all data are de-identified, all studies utilizing VDW data are approved as non-human subjects research by the Saint Louis University Institutional Review Board.
For this retrospective cohort study, we selected patients aged 18 or older with a new ICD-9 or ICD-10 diagnosis of either NICM or CA after January 1, 2011. The remaining ICD codes used for defining the studied population and outcomes are included in Supplementary Material 1 (cr.elmerpub.com). A list of ICD codes used to define the comorbidities is included in Supplementary Material 2 (cr.elmerpub.com). Patients were required to have evidence of healthcare activity in the 2 years prior to incident diagnosis and in the 6 months after diagnosis. This ensures that patients received their care primarily in the SSM system and available data were representative of care received. Patients on transthyretin stabilizers, i.e., tafamidis were not included in this analysis. NICM patients were matched 2:1 to CA patients based on age, gender, and race.
The primary exposure variable was the cardiac diagnosis: NICM or CA. Hospitalization with a HF diagnosis was identified based on inpatient encounter data with a corresponding primary diagnosis of HF. Ventricular tachyarrhythmia (VTA) episodes were identified using ICD-9 and ICD-10 codes and included ventricular tachycardia or ventricular fibrillation. Implantable cardiac defibrillator (ICD) and pacemaker (PM) placement were defined based on procedure codes (CPT, procedure ICD-9, and procedure ICD-10). Covariates included age at diagnosis, race, and comorbid medical conditions. Detailed definitions for all variables are in Supplementary Materials 1, 2 (cr.elmerpub.com).
Bivariate associations were assessed using Student’s t-tests for continuous variables and Chi-square and Fisher’s exact tests for categorical variables. Separate logistic regression models were computed to estimate the association between cardiac diagnosis and odds of each outcome. Kaplan-Meier curves were used to compare the time to outcome event for selected outcomes. An alpha value of 0.05 was used for all tests and SAS v9.4 (Cary, NC) was used for all analyses.
| Results | ▴Top |
Patient characteristics are listed in Table 1. After matching, there were 231 and 462 patients with CA and NICM, respectively, who fit our inclusion criteria. CA patients had higher prevalence of peripheral vascular disease (48.5% vs. 35.5%) and coronary artery disease (10.4% vs. 6.1%) at the time of diagnosis. Patient labs and cardiac medications are listed in Table 2. Significantly more CA patients were on angiotensin-converting enzyme (ACE) inhibitors (26.8% vs. 19.9%), angiotensin receptor blockers (75.8% vs. 62.1%), calcium channel blockers (61.9% vs. 44.6%), statin (74.9% vs. 60.2%) and diuretics (82.7% vs. 74.7%), compared to NICM patients. Mean follow-up duration was 48.6 vs. 44.3 months, respectively.
 Click to view | Table 1. Patient Demographics and Clinical Characteristics at Baseline |
 Click to view | Table 2. Basic Laboratory Values and Cardiac Medications |
Clinical outcomes are listed in Table 3. CA patients had significantly higher rates of HF hospitalization (HFH, 57.6% vs. 46.1%) and lower rates of ICD placement (1.7% vs. 5.9%). CA had a significantly higher rate of right bundle branch blocks (10.3% vs. 3.3%). The logistic regression model data are listed in Table 4. In the unadjusted data, CA patients had higher odds for HFH (odds ratio (OR): 1.58, 95% confidence interval (CI): 1.15 - 2.68) and lower odds for an ICD placement (OR: 0.28, 95% CI: 0.10 - 0.81). There was no significant difference in the odds of VTA (OR: 0.85, 95% CI: 0.51 - 1.41) or receiving a PM implantation (OR: 0.66, 95% CI: 0.35 - 1.24). After adjusting for demographics and relevant comorbidities, CA patients still had higher odds of HFH (OR: 1.86, 95% CI: 1.29 - 2.68). HF etiology (CA vs. NICM) remained unassociated with VTA (OR: 0.78, 95% CI: 0.44 - 1.4) or PM (OR: 0.64, 95% CI: 0.24 - 1.68) after adjusting for covariates. Due to the small number of patients receiving ICD in the CA group, adjusted logistic models were not possible for this outcome.
 Click to view | Table 3. Cardiac Outcomes for CA vs. NICM Patients |
 Click to view | Table 4. Adjusted Logistic Regression |
In the unadjusted survival analysis, there was no significant difference in the hazard ratio (HR) for HFH (HR: 1.21, 95% CI: 0.93 - 1.56) or VTA (HR: 0.69, 95% CI: 0.38 - 1.23). In the adjusted survival analysis, CA patients had a trend for a higher HR for HFH (HR: 1.31, 95% CI: 0.97 - 1.75), lower VTA events (HR: 0.53, 95% CI: 0.25 - 1.11) and receiving a PM (HR: 0.56, 95% CI: 0.17 - 1.85). Kaplan-Meier survival curves (Figs. 1-4) showed a trend for HFH to occur sooner, and PM or ICD to be implanted later from the diagnosis in CA patients.
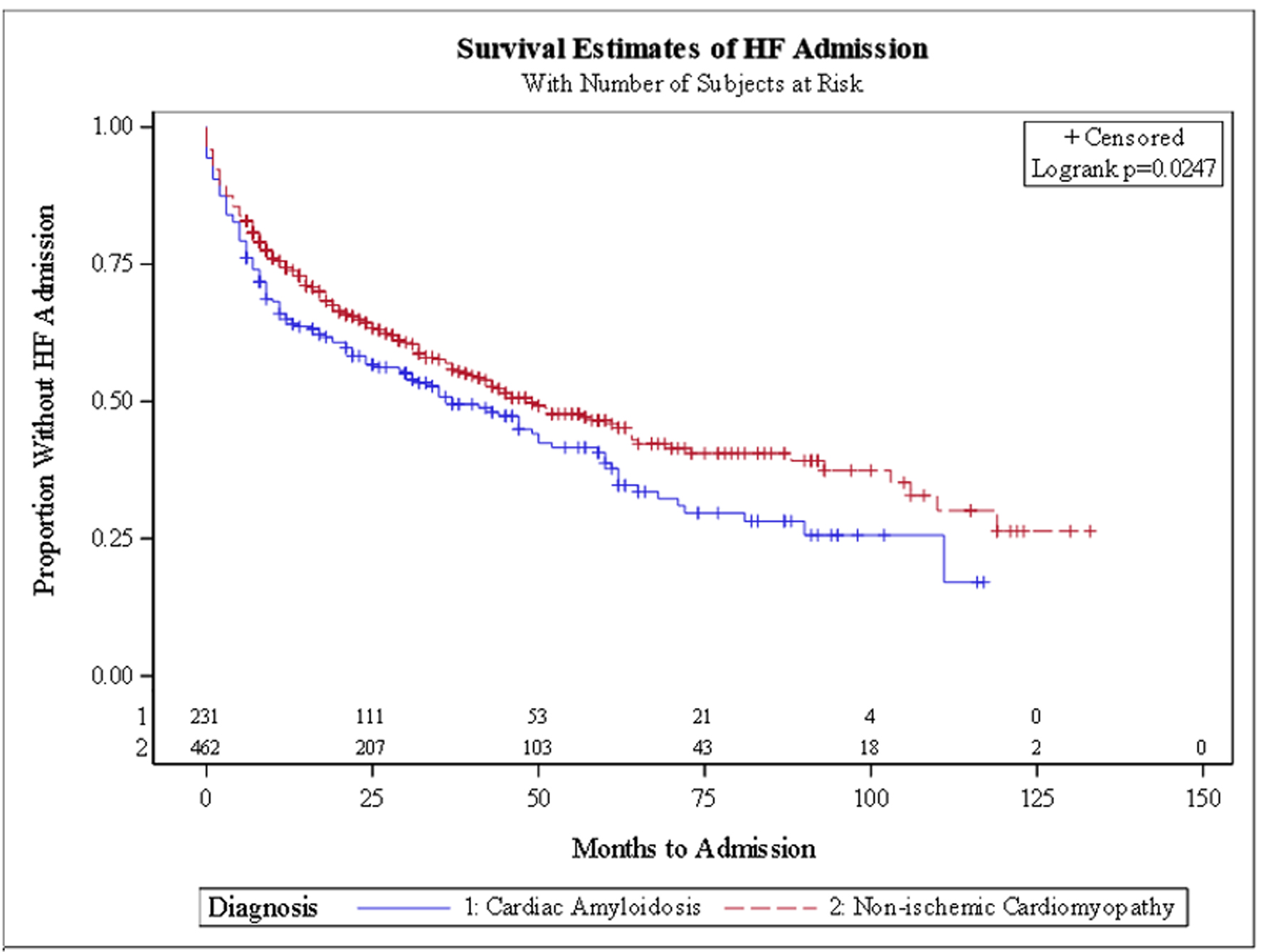 Click for large image | Figure 1. Kaplan-Meier curve for heart failure hospitalization. |
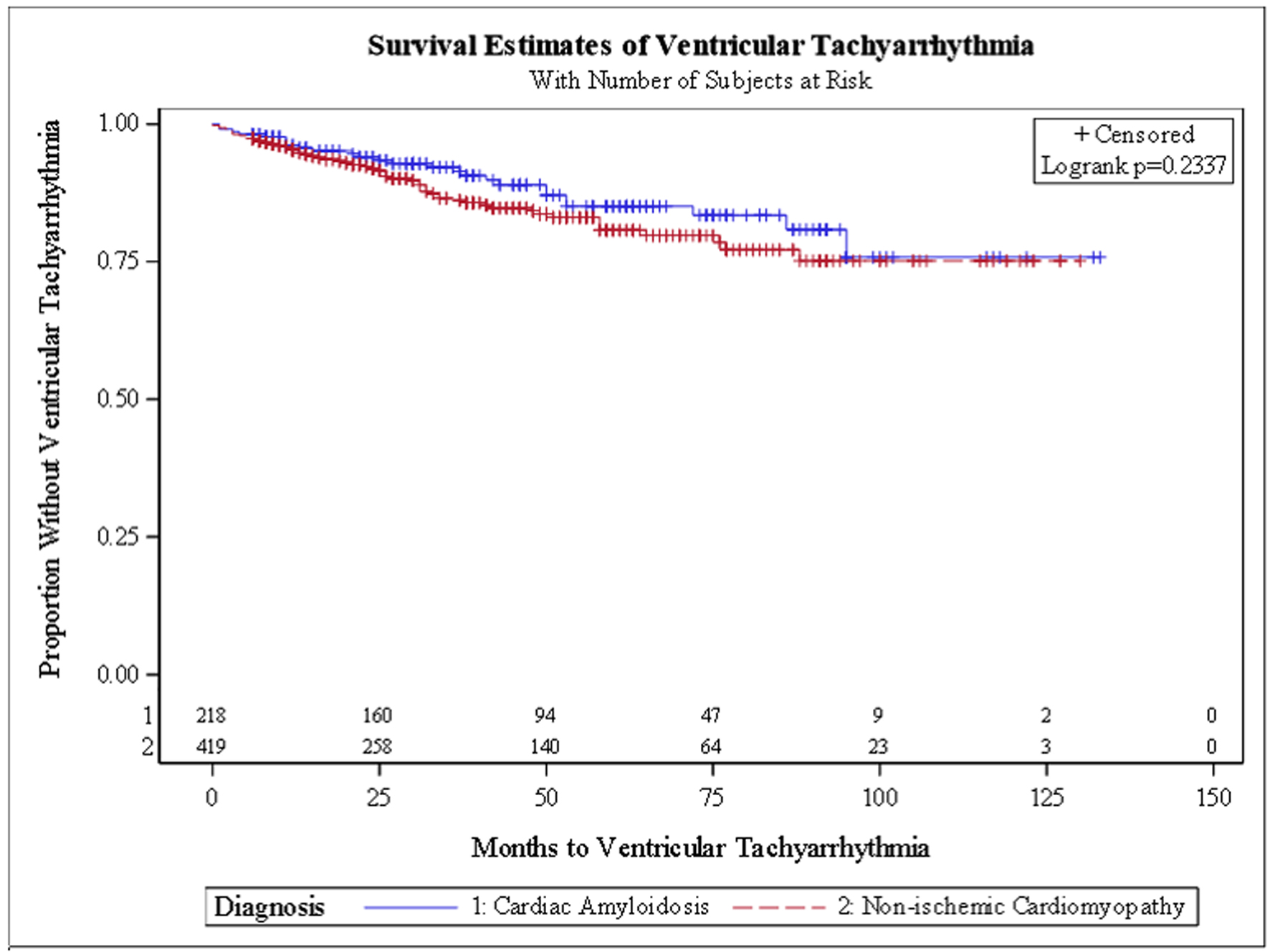 Click for large image | Figure 2. Kaplan-Meier curve for ventricular tachyarrhythmia. |
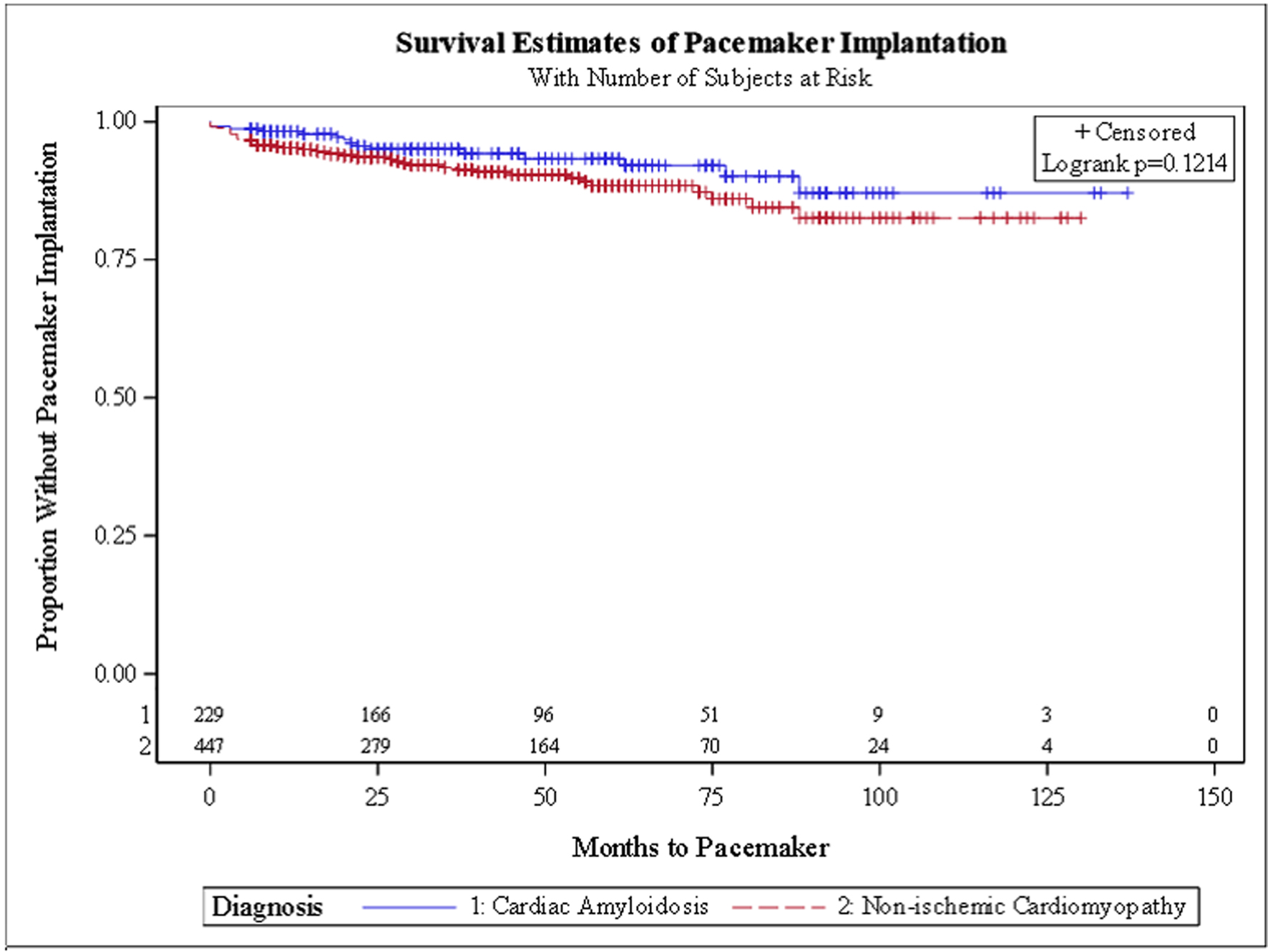 Click for large image | Figure 3. Kaplan-Meier curve for pacemaker placement. |
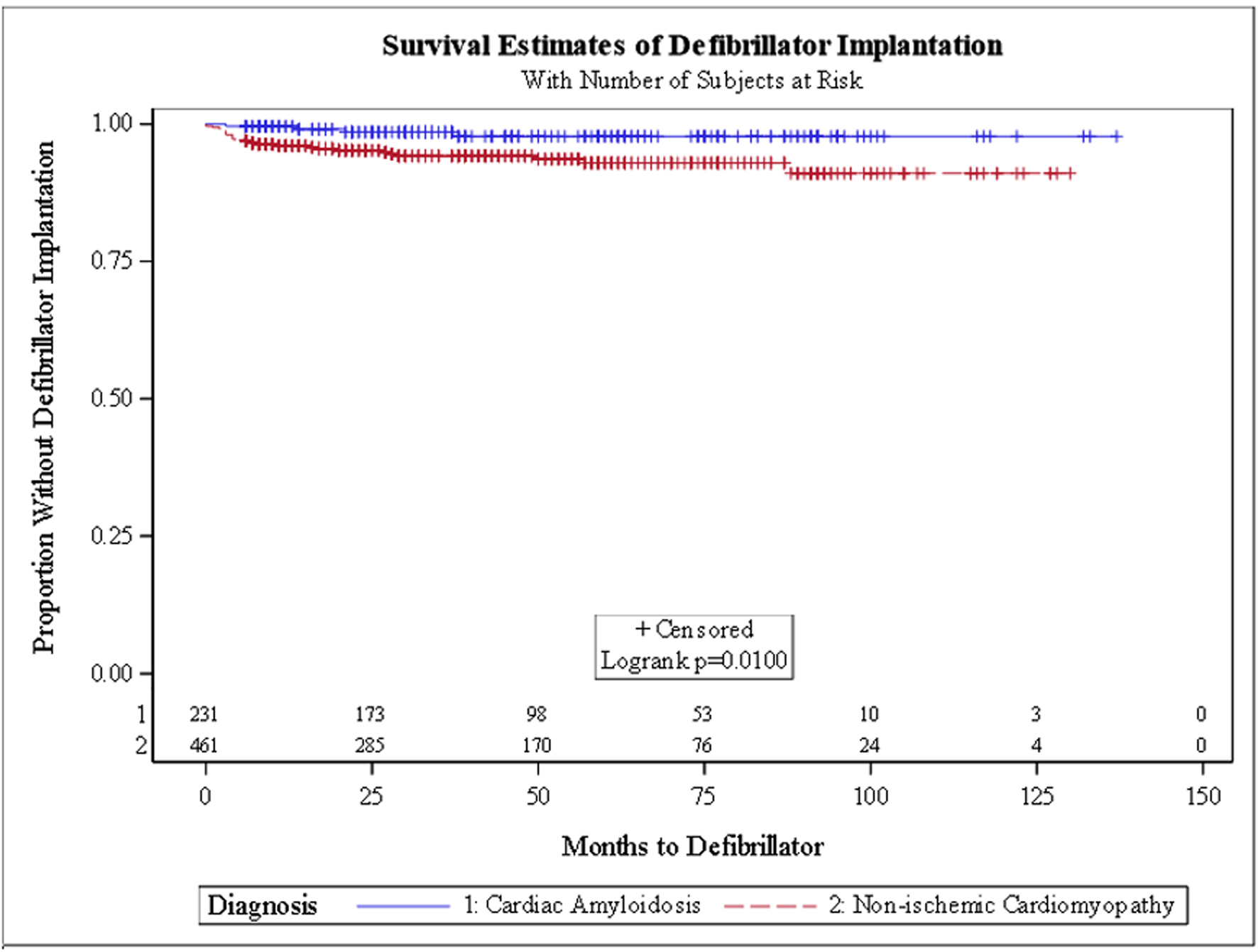 Click for large image | Figure 4. Kaplan-Meier curve for defibrillator placement. |
| Discussion | ▴Top |
In this retrospective study from a large healthcare system database, our main findings were as follows. 1) Patients with transthyretin CA had significantly higher odds of HFHs. 2) CA patients tended to have lower rates of VTA initially but similar rates overall. 3) There was a trend for CA patients to receive a PM or ICD later from the diagnosis.
Our results are generally in line with prior studies. Our studied population with transthyretin CA had similar demographic, comorbidity and laboratory profile to prior studies, including elevated B-type natriuretic peptide and troponin levels, which have been shown to be markers of poor prognosis [9-11]. Of note, in our study, right bundle branch block was more prevalent in CA patients and this finding has also been shown in previous studies [12]. Also of note, a relatively large percentage of patients in both groups were on calcium channel blockers even though societal guidelines recommend caution using them in CA patients [13].
CA patients in our study had significantly higher rates of HFHs, with a trend to occur earlier from the date of diagnosis. This is in line with prior studies that showed significant recurrent HFHs and decreased survival in CA patients [14]. Patients with ATTR-CA may experience more frequent and severe heart failure exacerbations than those with NICM due to the specific pathophysiology of CA. In ATTR-CA, amyloid fibrils, primarily derived from misfolded transthyretin (TTR) proteins, accumulate in the myocardium, leading to restrictive cardiomyopathy, stiffening of the heart walls, and impaired diastolic function [15]. Additionally, the amyloid deposition contributes to conduction system abnormalities, increasing the risk of arrhythmias, which can further exacerbate heart failure [16, 17]. The progressive nature of amyloid deposition, particularly when left untreated, leads to more frequent hospitalizations due to decompensated heart failure compared to NICM, where myocardial dysfunction may progress more slowly, often without the added burden of systemic amyloid-related organ involvement [18, 19]. In a propensity-matched analysis from the National Inpatient Sample between 2005 and 2014, patients admitted with CA had a longer length of stay (7.5 vs. 6.2 days), were less likely to be discharged home (43.6% vs. 48.7%) and were more likely to die during the hospitalization (7.4% vs. 4.9%, P < 0.001 for all) [20]. Tafamidis, which prevents cleavage of transthyretin tetramers and may reduce deposition of amyloid, has been approved for patients with TTR CA after significantly reducing mortality and cardiovascular-related hospitalizations in the ATTR-ACT study [19]. Patients on tafamidis were not included in this study and this analysis would be a good reference as ATTR stabilizers, among other newer therapies, are expected to change the landscape of TTR CA in the near future [21], once they have been widely utilized [22-24].
The relatively high rate of PM needed in both groups in our study is in line with prior studies. In ATTR-CA, the deposition of amyloid fibrils in the myocardium disrupts the normal structure and function of the heart, particularly the conduction system. This deposition can cause electrical conduction abnormalities such as atrioventricular (AV) block, bundle branch block, and other arrhythmias, which significantly increase the risk of requiring a PM [17]. Furthermore, amyloid infiltration of the heart muscle can lead to stiffness and restrictive cardiomyopathy, which impairs diastolic filling and increases the risk of ventricular arrhythmias, including those that can trigger sudden cardiac death. The VTA survival curves in our study initially showed a trend for VTA to occur later in CA patients but overall the incidence was similar to NICM; this finding is similar to prior studies [13, 25]. CA patients are known to be at high risk for electromechanical dysregulation, and arrhythmias in CA patients are associated with increased mortality, acute HF exacerbations, increased length of stay, hospitalization costs [6] and sudden cardiac death [26]. Interestingly, even though the overall incidence of VTA was similar between both groups, CA patients had less ICD devices placed. The guidelines for device placement for primary prevention in CA patients are rather unclear [26, 27]. There is also a paucity of data showing mortality benefit to primary prevention utilizing ICD implantation in CA patients and some case reports and small observational studies even suggested that it may be harmful [28, 29]. This might explain the lower rate of device placements in CA patients in our study [17, 30-32].
There are several limitations to our study. Because of the retrospective methodology using an administrative database, we did not have data on key clinical indicators of antecedent disease severity including previous numbers of hospitalizations. We were not able to include mortality data in our analysis due to the de-identified nature of the data in the VDW. In addition, details of management were also unavailable including therapies during hospitalization, efficiency of decongestion during hospitalization and dosage optimization of guideline-directed medical therapies as outpatient, all of which may potentially have affected the course and future hospitalizations. As we used a database limited to the hospitals in the SSM network, any care received outside of this hospital system was not captured and not available for analysis and interpretation, leading to an underestimation of the results. This is somewhat mitigated by us including only patients with a history of follow-up in our system. Finally, we could not evaluate other pertinent variables such as the diagnostic criteria used to diagnose the comorbid conditions, and outcomes. The strengths of this study include a significant number of CA patients who were assessed across multiple hospitals despite the somewhat rare nature of the disease. Further studies should assess how the management of the medical comorbidities in this study affect overall mortality on patients CA. In conclusion, in this retrospective study from a large healthcare system database, compared to NICM, transthyretin CA patients had significantly higher odds of HFHs and lower odds of receiving ICD. There was a trend for CA patients to develop HFH sooner and receive a PM or ICD later from the diagnosis.
| Supplementary Material | ▴Top |
Suppl 1. ICD codes used to define the studied population and outcomes.
Suppl 2. ICD codes used for defining comorbidities.
Acknowledgments
None to declare.
Financial Disclosure
The authors did not receive any funding for this work.
Conflict of Interest
The authors do not have any conflict of interest for this work.
Informed Consent
The authors have no relevant relationships with industry to disclose. This research was completed using a deidentified database that did not require informed consent and was approved by the Institutional Review Board at our institution.
Author Contributions
Barbara Okeke and Mina Benjamin are responsible for drafting the manuscript and Timothy Chrusciel is responsible for the statistical analysis.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
ACE: angiotensin-converting enzyme; CA: cardiac amyloidosis; HCSRN: Health Care Systems Research Network; HF: heart failure; ICD: implantable cardiac defibrillator; PM: pacemaker; SCD: sudden cardiac death; VDW: virtual data warehouse; VTA: ventricular tachyarrhythmia
| References | ▴Top |
- de Marneffe N, Dulgheru R, Ancion A, Moonen M, Lancellotti P. Cardiac amyloidosis: a review of the literature. Acta Cardiol. 2022;77(8):683-692.
doi pubmed - Writing C, Kittleson MM, Ruberg FL, Ambardekar AV, Brannagan TH, Cheng RK, Clarke JO, et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: a report of the american college of cardiology solution set oversight committee. J Am Coll Cardiol. 2023;81(11):1076-1126.
doi pubmed - Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. 2018;2(10):1046-1053.
doi pubmed - Gilstrap LG, Dominici F, Wang Y, El-Sady MS, Singh A, Di Carli MF, Falk RH, et al. Epidemiology of cardiac amyloidosis-associated heart failure hospitalizations among fee-for-service medicare beneficiaries in the United States. Circ Heart Fail. 2019;12(6):e005407.
doi pubmed - Tan NY, Mohsin Y, Hodge DO, Lacy MQ, Packer DL, Dispenzieri A, Grogan M, et al. Catheter ablation for atrial arrhythmias in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol. 2016;27(10):1167-1173.
doi pubmed - Thakkar S, Patel HP, Chowdhury M, Patel K, Kumar A, Arora S, Zahid S, et al. Impact of arrhythmias on hospitalizations in patients with cardiac amyloidosis. Am J Cardiol. 2021;143:125-130.
doi pubmed - Porcari A, Fontana M, Gillmore JD. Transthyretin cardiac amyloidosis. Cardiovasc Res. 2023;118(18):3517-3535.
doi pubmed - www.hcsrn.org.
- Oerlemans M, Rutten KHG, Minnema MC, Raymakers RAP, Asselbergs FW, de Jonge N. Cardiac amyloidosis: the need for early diagnosis. Neth Heart J. 2019;27(11):525-536.
doi pubmed - Aimo A, Januzzi JL, Jr., Vergaro G, Ripoli A, Latini R, Masson S, Magnoli M, et al. Prognostic value of high-sensitivity troponin T in chronic heart failure: an individual patient data meta-analysis. Circulation. 2018;137(3):286-297.
doi pubmed - Perfetto F, Bergesio F, Emdin M, Cappelli F. Troponins in cardiac amyloidosis: multipurpose markers. Nat Rev Cardiol. 2014;11(3):179.
doi pubmed - See ASY, Ho JS, Chan MY, Lim YC, Yeo TC, Chai P, Wong RCC, et al. Prevalence and risk factors of cardiac amyloidosis in heart failure: a systematic review and meta-analysis. Heart Lung Circ. 2022;31(11):1450-1462.
doi pubmed - Barbhaiya CR, Kumar S, Baldinger SH, Michaud GF, Stevenson WG, Falk R, John RM. Electrophysiologic assessment of conduction abnormalities and atrial arrhythmias associated with amyloid cardiomyopathy. Heart Rhythm. 2016;13(2):383-390.
doi pubmed - Ladefoged BT, Dybro A, Dahl Pedersen AL, Rasmussen TB, Vase HO, Clemmensen TS, Gillmore J, et al. Incidence and predictors of worsening heart failure in patients with wild-type transthyretin cardiac amyloidosis. ESC Heart Fail. 2022;9(5):2978-2987.
doi pubmed - Coelho T, Maurer MS, Suhr OB. THAOS - The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29(1):63-76.
doi pubmed - Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC State-of-the-Art review. J Am Coll Cardiol. 2019;73(22):2872-2891.
doi pubmed - Siddiqi OK, Ruberg FL. Cardiac amyloidosis: An update on pathophysiology, diagnosis, and treatment. Trends Cardiovasc Med. 2018;28(1):10-21.
doi pubmed - McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004.
doi pubmed - Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016.
doi pubmed - Sperry BW, Saeed IM, Raza S, Kennedy KF, Hanna M, Spertus JA. Increasing rate of hospital admissions in patients with amyloidosis (from the National Inpatient Sample). Am J Cardiol. 2019;124(11):1765-1769.
doi pubmed - Swanton RH, Brooksby IA, Davies MJ, Coltart DJ, Jenkins BS, Webb-Peploe MM. Systolic and diastolic ventricular function in cardiac amyloidosis. Studies in six cases diagnosed with endomyocardial biopsy. Am J Cardiol. 1977;39(5):658-664.
doi pubmed - Gillmore JD, Judge DP, Cappelli F, Fontana M, Garcia-Pavia P, Gibbs S, Grogan M, et al. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy. N Engl J Med. 2024;390(2):132-142.
doi pubmed - Coelho T, Marques W, Jr., Dasgupta NR, Chao CC, Parman Y, Franca MC, Jr., Guo YC, et al. Eplontersen for hereditary transthyretin amyloidosis with polyneuropathy. JAMA. 2023;330(15):1448-1458.
doi pubmed - Masri A, Van Spall HGC. In adults with ATTR cardiac amyloidosis, patisiran reduced decline in functional capacity at 12 mo. Ann Intern Med. 2024;177(3):JC30.
doi pubmed - Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, Burazor I, et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur J Heart Fail. 2021;23(4):512-526.
doi pubmed - Bukhari S, Khan SZ, Ghoweba M, Khan B, Bashir Z. Arrhythmias and device therapies in cardiac amyloidosis. J Clin Med. 2024;13(5):1300.
doi pubmed - Varr BC, Zarafshar S, Coakley T, Liedtke M, Lafayette RA, Arai S, Schrier SL, et al. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm. 2014;11(1):158-162.
doi pubmed - Lin G, Dispenzieri A, Kyle R, Grogan M, Brady PA. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol. 2013;24(7):793-798.
doi pubmed - Higgins AY, Annapureddy AR, Wang Y, Minges KE, Lampert R, Rosenfeld LE, Jacoby DL, et al. Survival following implantable cardioverter-defibrillator implantation in patients with amyloid cardiomyopathy. J Am Heart Assoc. 2020;9(18):e016038.
doi pubmed - McNally EM, Mestroni L. Dilated cardiomyopathy: genetic determinants and mechanisms. Circ Res. 2017;121(7):731-748.
doi pubmed - Merlini G, Seldin DC, Gertz MA. Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol. 2011;29(14):1924-1933.
doi pubmed - Reisinger J, Dubrey SW, Lavalley M, Skinner M, Falk RH. Electrophysiologic abnormalities in AL (primary) amyloidosis with cardiac involvement. J Am Coll Cardiol. 1997;30(4):1046-1051.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.