| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 000, Number 000, April 2025, pages 000-000
Impact of Systematic Use of Fractional Flow Reserve and Optical Coherence Tomography on Percutaneous Coronary Intervention Outcomes in Patients With Diabetes
Charantharayil Gopalan Bahuleyana, l, Selvamani Sethuramanb, Fazila-Tun-Nesa Malikc, Sridhar Kasturid, Manoj Bhavarilal Chopdae, Rony Mathew Kadavilf, Rajneesh Kapoorg, Sanjeeb Royh, Rajpal Abhaichandi, Ajit Menonj, Vijayakumar Subbank
aCardiovascular Centre, Ananthapuri Hospital & Research Institute, Trivandrum, India
bDepartment of Cardiology, Meenakshi Mission Hospital & Research Centre, Madurai, India
cDepartment of Cardiology, National Heart Foundation Hospital & Research Institute, Dhaka, India
dDepartment of Cardiology, Sunshine Hospital, Hyderabad, India
eDepartment of Intervention Cardiology, Magnum Heart Institute, Nashik, India
fDepartment of Cardiology, Lisie Heart Institute, Lisie Hospital, Ernakulum, India
gInterventional Cardiology, Heart Institute, Medanta - The Medicity, Gurgaon, India
hDepartment of Cardiology, Cardiac Sciences, Manglam Plus Medicity Hospital, Jaipur, India
iDepartment of Cardiology, G. Kuppuswamy Naidu Memorial Hospital, Coimbatore, India
jDepartment of Cardiology, Lilavati Hospital & Research Centre, Mumbai, India
kIndian Cardiology Research Foundation, Core Lab, Chennai, India
lCorresponding Author: Charantharayil Gopalan Bahuleyan, Cardiovascular Centre, Ananthapuri Hospital & Research Institute, Thiruvananthapuram, Kerala 695024, India
Manuscript submitted January 27, 2025, accepted March 17, 2025, published online April 5, 2025
Short title: Impact of FFR and OCT on PCI Outcomes
doi: https://doi.org/10.14740/cr2052
| Abstract | ▴Top |
Background: Intracoronary imaging and physiology guidance of percutaneous coronary intervention (PCI) have shown significant improvements in clinical outcomes. However, comparable data on the use of these modalities in PCI of patients with diabetes are only sparsely available from South Asia. This study investigated the feasibility and clinical outcomes of systematic use of fractional flow reserve (FFR) and optical coherence tomography (OCT) during PCI in patients with diabetes.
Methods: The study enrolled 275 patients (≥ 18 years) from nine centers in India and one from Bangladesh between October 2021 and September 2022. Patients with stable ischemic heart disease, non-ST-elevation myocardial infarction (MI), and unstable angina were included in the study. Angiographically intermediate lesions (diameter stenosis of 40% to 80%) underwent FFR-guided PCI. Lesions with a diameter stenosis of > 80% underwent PCI without FFR evaluation. All PCI procedures were guided by OCT using the MLD-MAX algorithm.
Results: At 12 months, the target lesion failure (TLF) rate, a composite of cardiac death, nonfatal MI, and clinically driven target lesion revascularization, was 3.3%. Among the intermediate lesions, PCI was deferred by 70% after the FFR evaluation. Pre- and post-procedural OCT has led to a strategy change in 49.5% and 33.6%, respectively.
Conclusions: The study revealed a relatively lower rate of events with FFR and OCT guidance compared to historical data from angiography-guided PCI in patients with diabetes. The strategy of combined use of FFR and OCT in PCI may contribute to improved clinical outcomes in patients with diabetes.
Keywords: Diabetes mellitus; Physiology; Optical coherence tomography; Fractional flow reserve
| Introduction | ▴Top |
Diabetes mellitus (DM) is a significant global health crisis, and in particular it has emerged as a major public health challenge in South Asia [1]. Coronary artery disease (CAD) is very common in patients with diabetes, often associated with multivessel and diffuse disease, lesions being more complex, revealing a higher number of larger calcium arc and calcium nodules [2, 3]. A real-world registry (N = 43,209 patients) has shown that diabetes is independently associated with increased early stent thrombosis, mortality, and major adverse cardiac events (MACE) [4].
Integrating coronary physiology and imaging in percutaneous coronary intervention (PCI) has resulted in a reduction of MACE, including mortality. Randomized controlled studies, registries, and large meta-analyses supported physiology-guided PCI over angio-guided PCI [5-8]. However, the impact of physiology-guided PCI in patients with diabetes was variable in clinical studies [9, 10]. Intracoronary imaging (ICI) (intravascular ultrasound (IVUS), optical coherence tomography (OCT)) guided PCI is associated with a reduced risk of myocardial infarction (MI), target vessel revascularization (TVR), stent thrombosis, and mortality compared to angiography-guided PCI [11-14]. OCT with its higher resolution capacity provides accurate vessel measurements, plaque characteristics, and stent details, which are essential prerequisites to perform optimal PCI. However, studies on the systematic use of fractional flow reserve (FFR) and OCT for optimizing PCI in patients with diabetes are lacking.
This study involving nine leading cardiac intervention centers in India and one in Bangladesh assessed the feasibility and clinical outcomes of employing a strategy of combined use of FFR and OCT in PCI of patients with diabetes. Intermediate lesions (a diameter stenosis of 40-80%) underwent PCI based on FFR value of ≤ 0.80 and severe lesions > 80% without FFR evaluation. All PCI procedures were guided by OCT using the MLD-MAX algorithm [15].
| Materials and Methods | ▴Top |
Study design and population
This prospective, multicenter study enrolled 275 patients with DM from 10 different clinical sites between October 2021 and September 2022 to evaluate the potential benefits of using OCT and FFR on the outcomes of PCI. The study commenced following ethics committee approval from the respective institutes and was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. Written informed consent was obtained from all the study participants.
Patients of either gender with diabetes, aged ≥ 18 years, diagnosed with stable ischemic heart disease, non-ST-segment elevation myocardial infarction, or unstable angina, and presenting with single or multivessel lesions eligible for PCI with FFR and/or OCT guidance were included. Patients with hemodynamic instability or cardiogenic shock, with a life expectancy of less than 12 months, contraindications for dual antiplatelet therapy, chronic total occlusion or aorto-ostial lesions, previous coronary bypass artery grafts, eligibility for primary PCI due to ST-segment elevation myocardial infarction, vessel size < 2.5 mm or > 4 mm, in-stent restenosis lesions, left main disease, glomerular filtration rate < 30 mL/min, Killip class III or IV, difficult-to-study distal coronary lesions for OCT or FFR, or lesions where FFR and/or OCT could not be performed due to technical limitations were excluded. The study design is shown in Figure 1.
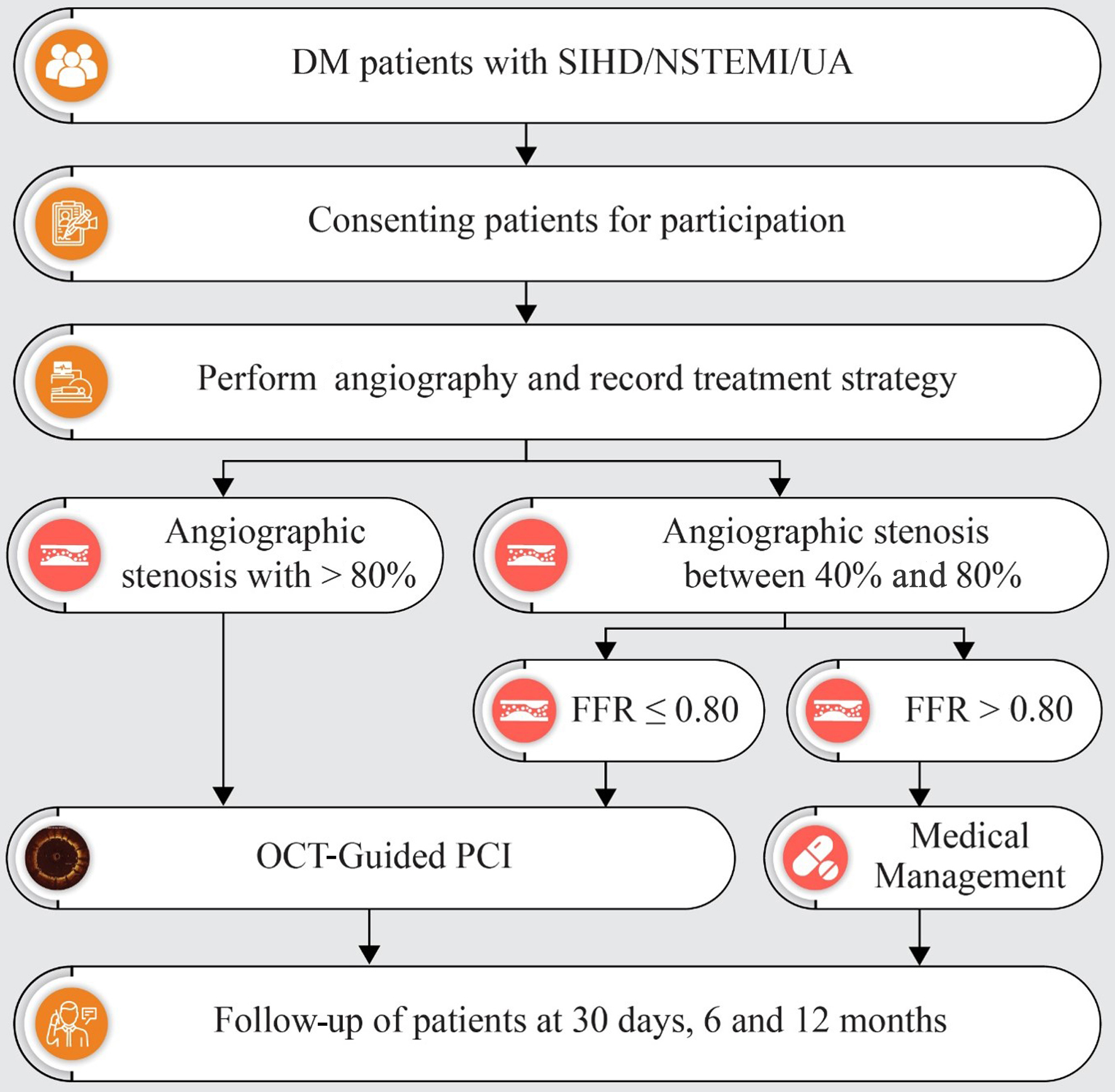 Click for large image | Figure 1. Flowchart depicting the study plan and follow-up. DM: diabetes mellitus; FFR: fractional flow reserve; NSTEMI: non-ST-segment elevation myocardial infarction; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; SIHD: stable ischemic heart disease; UA: unstable angina. |
Methodology
The patients underwent coronary angiography following the standard protocol. The lesion complexity was classified based on the American Heart Association (AHA)/American College of Cardiology (ACC) consensus statement [16]. SYNTAX score was also calculated for all patients. Intermediate lesions (a diameter stenosis of 40-80%) were evaluated by physiological assessment using Pressure Wire™ X (Abbott Vascular, USA). The resting distal coronary artery pressure/aortic pressure and the resting full-cycle ratio (RFR) were recorded first followed by FFR estimation after achieving maximum hyperemia with intravenous adenosine. The treatment strategy was decided based on the FFR cutoff value of 0.80.
Lesions with stenosis > 80% and FFR-positive (≤ 0.80) intermediate lesions underwent OCT-guided PCI. OCT imaging was performed using a Dragonfly OPTIS™ imaging catheter (Abbott Vascular, Santa Clara, CA, USA). The OCT catheter was introduced into the vessel over the 0.014-inch guidewire, and images were acquired after the administration of intracoronary nitrate as required. All vessels were cleared of blood by injecting a contrast medium. All necessary optimizations were carried out based on post-stenting OCT findings. Two runs of OCT, i.e., pre- and post-PCI, were performed in all patients, and a third or final run of OCT was also performed in cases where there was an optimization after the second run. Those with an FFR value > 0.80 were prescribed optimal medical treatment at the investigators’ discretion. Angiographic, physiologic, and OCT images were reviewed by the Core Lab (Indian Cardiology Research Foundation, Chennai, India), and the analysis was based on Core Lab data. Morphology was analyzed in detail to identify various plaque features, such as layered, calcified, fibrous, lipid-rich, thin-cap fibroatheroma, plaque erosion, plaque rupture, macrophages, microchannels, cholesterol crystals, calcified nodule, and spotty calcium. A lesion with the presence of three or more vulnerable features was categorized as high-risk plaque (HRP). The patients were followed up at 1, 6, and 12 months. Details regarding the patient’s well-being and any hospitalization or revascularization after the initial PCI were recorded during follow-up.
Endpoints
The primary endpoint was the rate of target lesion failure (TLF), i.e., a composite of cardiac death, nonfatal MI, and ischemia-driven target lesion revascularization at 12 months. The secondary endpoints comprised: 1) assessing procedural efficiency (i.e., procedure time and contrast volume); 2) stent thrombosis; 3) rate of concordance and discordance between FFR and RFR (concordance is defined as FFR ≤ 0.80 and RFR ≤ 0.89 or FFR > 0.80 and RFR > 0.89, and discordance is defined as FFR > 0.80 and RFR ≤ 0.89 or FFR ≤ 0.80 and RFR > 0.89); and 4) clinical outcomes in concordant and discordant groups.
Sample size and statistical analysis
In previous studies of angiography-guided PCI in patients with diabetes, the TLF rate was found to be approximately 8% [17, 18]. To determine the clinical acceptance threshold, a margin of 4.5% was added. This margin aligns with the noninferiority criteria used in previous studies and the FDA guidelines established in 2010 [19], setting the clinical acceptance criterion at 12.5%. It is anticipated that the use of OCT and, where applicable, FFR-guided strategies will help achieve TLF rates of 9% or lower in this study. This rate was below the established clinical acceptance criterion of 12.5%, which was based on a one-sided 95% confidence interval (CI). The study aimed to include 255 subjects to statistically validate this outcome. Factoring in a dropout rate of 5%, the total number of participants required increased to 269. Therefore, the plan is to enroll approximately 275 patients to ensure robustness in the study’s findings.
Categorical variables are presented as numbers (percentage), while continuous data are expressed as mean ± standard deviation. TLF rates and procedural efficiency (time and contrast volume) are summarized using descriptive statistics. Lesion assessment by angiography and OCT was compared using a Chi-square test and t-test. A P-value of < 0.05 was considered to be statistically significant.
| Results | ▴Top |
Baseline characteristics
The study enrolled 275 patients; one patient was excluded due to a scheduled coronary artery bypass grafting procedure. In the study population, a total of 353 lesions were analyzed. The mean age was 59.1 ± 9.4 years, with 74% males. The baseline clinical characteristics of the study population and different indications for PCI are summarized in Table 1.
 Click to view | Table 1. Baseline Characteristics of the Study Population |
Lesion description by angiographic assessment
Most of the lesions were found in the left anterior descending and diagonal artery (n = 209). In terms of lesion types, the most prevalent were long lesions (61.7%) and bifurcation lesions (26.6%). Moderate-to-severe calcification was reported in 17% of the patients. About 61.4% of the lesions were found in small vessels (≤ 3 mm). Multivessel disease was diagnosed in 52.2% of the patients. According to the ACC/AHA classification, 87.3% of the lesions were categorized as type B and type C lesions. Severe lesions (a diameter stenosis of > 80%) and intermediate lesions (a diameter stenosis of 40-80%) were found in 76.8% and 23.2%, respectively (Fig. 2). More information about the lesions is shown in Table 2.
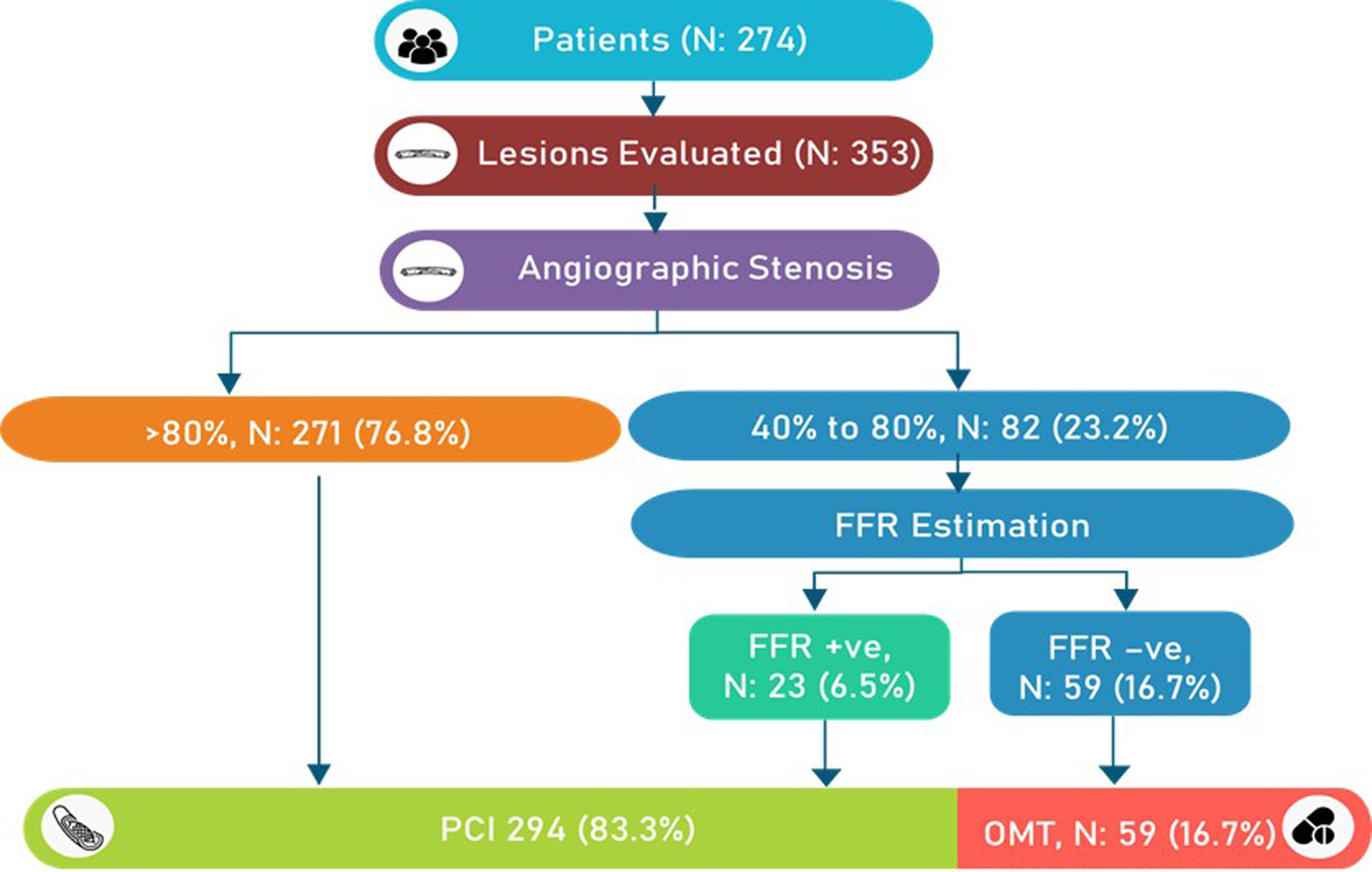 Click for large image | Figure 2. Flowchart with the number of lesions and procedural details. FFR: fractional flow reserve; OMT: optimal medical therapy. |
 Click to view | Table 2. Lesion Description |
Impact of physiology guidance in intermediate lesions (40-80%)
Of 82 intermediate lesions, 77 (93.9%) lesions were considered for PCI by angiographic assessment by investigators. However, after FFR evaluation, only 25 lesions (30.5%) received PCI (Fig. 3). It was seen that physiological assessment had a great impact on treatment decisions: in about 69.5% (57/82) of the intermediate lesions, intervention was deferred. If the decision were to be made by the RFR value, the deferral rate would have been 61%. The rate of concordance between the FFR and RFR values was 91.5% (Fig. 3).
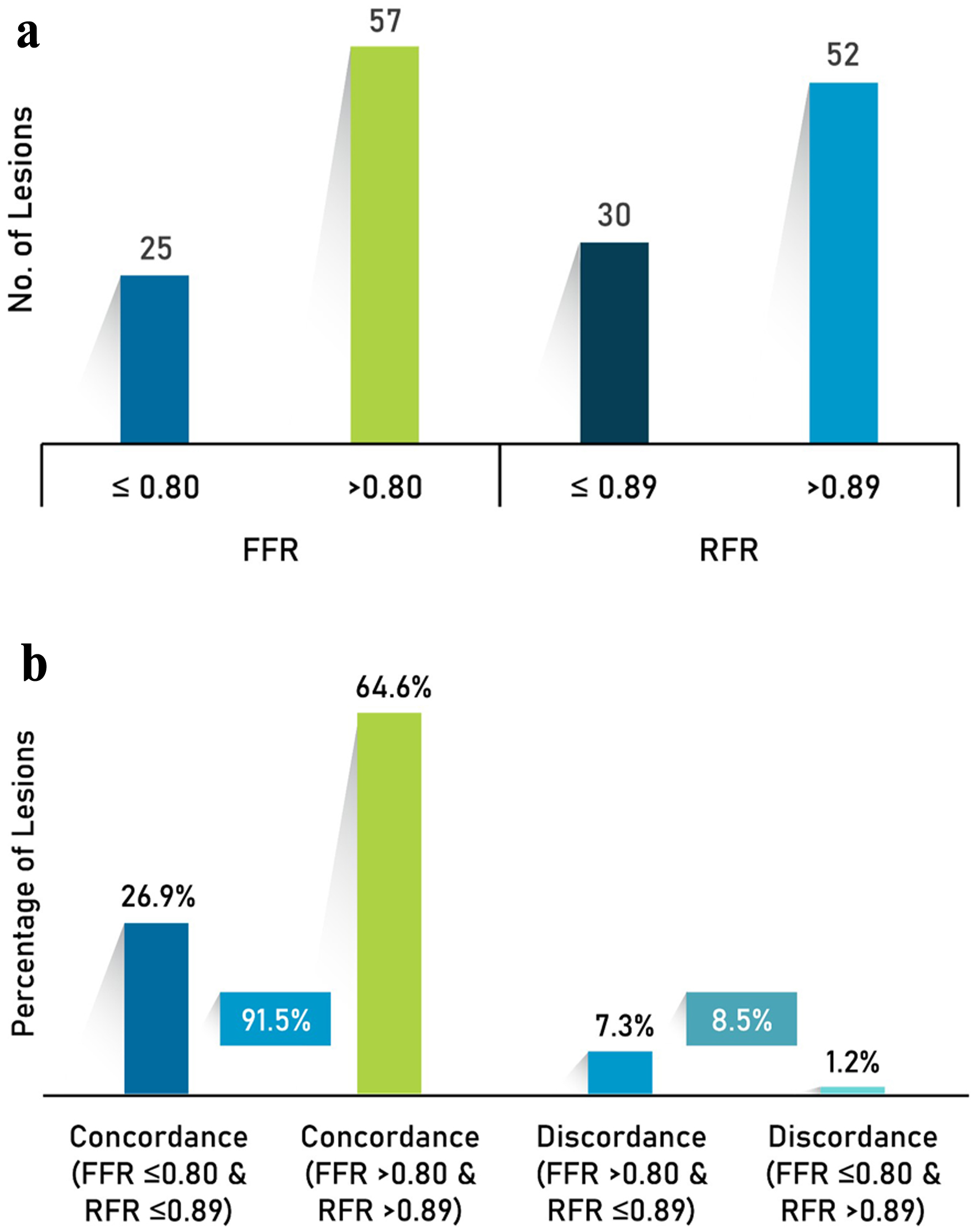 Click for large image | Figure 3. (a) Physiology assessment and (b) concordance and discordance between RFR and FFR. FFR: fractional flow reserve; RFR: resting full-cycle ratio. |
Pre-PCI assessment of lesions - angiography vs. OCT
The need for debulking was planned in 23.2% by angiography vs. 29.4% after OCT imaging. Calcification was detected in 36.6% of the lesions by angiography, whereas OCT detected calcium in 47.8% of the same set of lesions. A calcium score of 0 was observed in 52% of the lesions, followed by a calcium score of 2 and 4 in 20% and 16% of the lesions, respectively. The mean calcified plaque arc was 155.8 ± 110.0°. Statistically significant differences were observed between the angiographic and OCT groups in vessel dimensions (proximal reference vessel diameter (RVD): 3.2 ± 0.6 vs. 3.3 ± 0.6, and distal RVD: 2.8 ± 0.5 vs. 2.9 ± 0.6), lesion length (29.4 ± 13.8 vs. 32.0 ± 13.7), intended stent length (33.7 ± 14.6 vs. 35.5 ± 14.2), and diameter (2.9 ± 0.4 vs. 3.0 ± 0.5) during the pre-PCI assessment of lesions. There were no significant differences in the intended number of stents between these two groups (Table 3).
 Click to view | Table 3. Lesion Assessment by Angiography vs. OCT (n = 289 Lesions) |
OCT evaluation of plaque characteristics
The morphological details of the plaque at the minimal lumen area (MLA) and the total length of the lesion were analyzed by the Core Lab. Layered plaques (32.6%) followed by calcified and fibrous plaques (24% and 16%, respectively) were observed at the MLA site. A lesion with three or more HRP characteristics was found to be 53%. A high prevalence of macrophage accumulation (80.6%) and the presence of cholesterol crystals (34.4%) were observed in the total length of lesions.
Procedural details
The mean stent length was 35.5 ± 14.1 mm, and the mean diameter was 3.0 ± 0.5 mm. One stent was used in 90.9% of the lesions, and the mean number of stents was 1.1 ± 0.3 per lesion. Other procedural details include pre-dilatation in 85.3% of the lesions, and the lesion preparation was carried out using non-compliant balloons, specialty balloons, and atherectomy devices based on the plaque details revealed by OCT. Post-dilatation with a noncompliant balloon was performed in 97.6% of the lesions and by a compliant balloon in 0.7% of the lesions. Post-PCI angiographic evaluation revealed a residual percent diameter stenosis of 4.8±5.3%.
Post-PCI optimization
Post-PCI optimization strategies such as additional ballooning and/or stenting were performed in cases where stent underexpansion (n = 51), stent malapposition (n = 29), edge dissection (n = 20), incomplete lesion coverage (n = 3), and tissue prolapse (n = 36) were observed (Fig. 4).
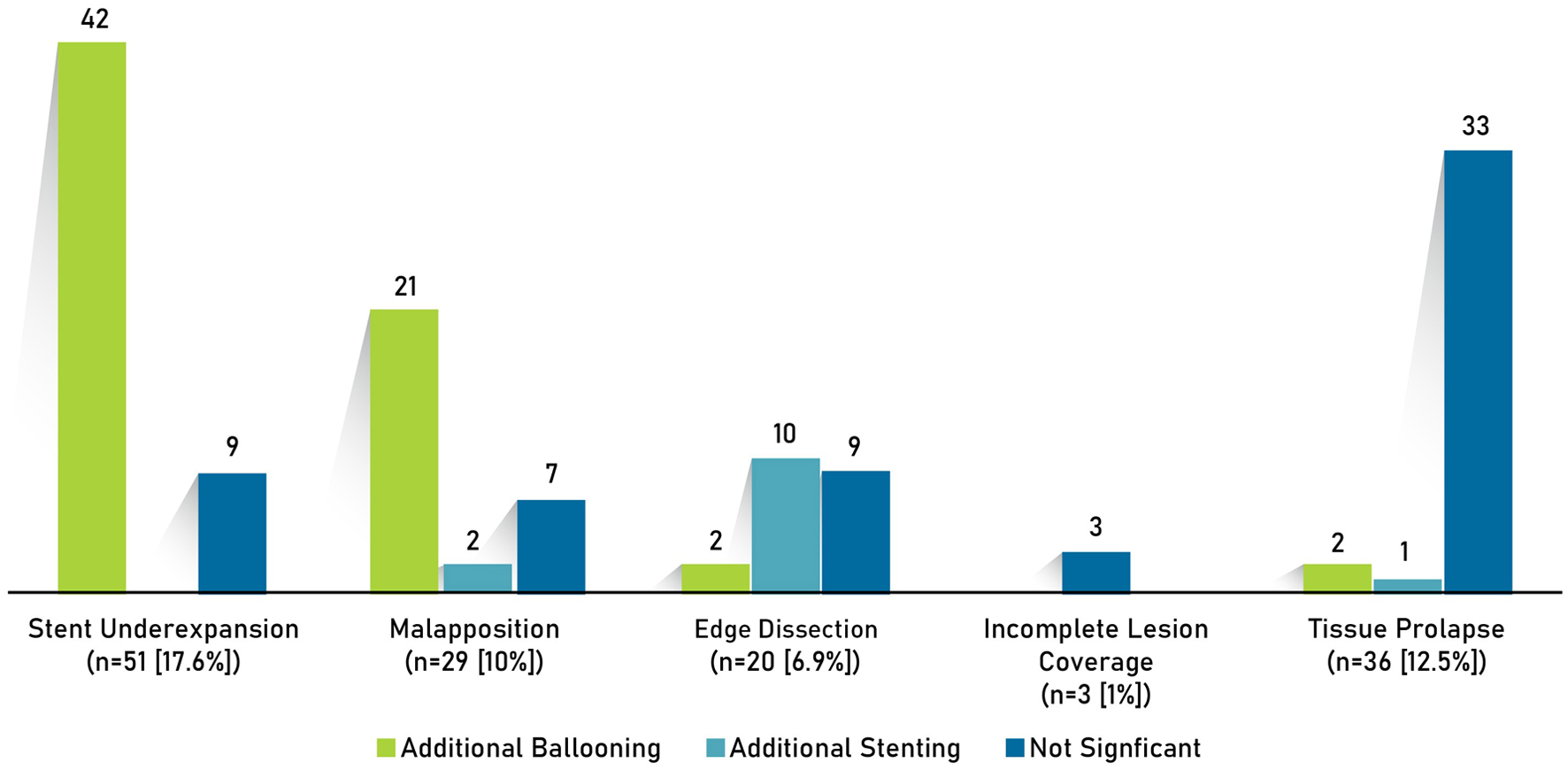 Click for large image | Figure 4. Post-PCI optimization strategies. PCI: percutaneous coronary intervention. |
The average minimum stent area (MSA) achieved in the study population was 6.1 ± 2.1 mm2. The average MSA was 5.2 ± 1.4 mm2 in small vessels (≤ 3 mm) and 8.2 ± 2.2 mm2 in large vessels (> 3 mm). As per the recommendation of the European Association of Percutaneous Cardiovascular Interventions Consensus 2018, an MSA of at least 4.5 mm2 was achieved in 99% of large vessels and 3.5 mm2 in 92% of small vessels.
Impact of OCT on PCI strategy
Preprocedural OCT influenced the PCI strategy in 49.5% of the cases (Fig. 5), inclusive of the need for lesion preparation (6%), change in stent length (34%), change in stent diameter (25%), and change in stenting strategy (5%). The overall cumulative OCT impact was 63%. The mean lesion length was 27.2 ± 13.6 mm. Postprocedural OCT resulted in changes comprising incomplete lesion coverage (1.2%), stent underexpansion (18%), stent malapposition (10%), stent edge dissection (7%), and tissue thrombus/prolapse (12%), which required post-PCI optimization.
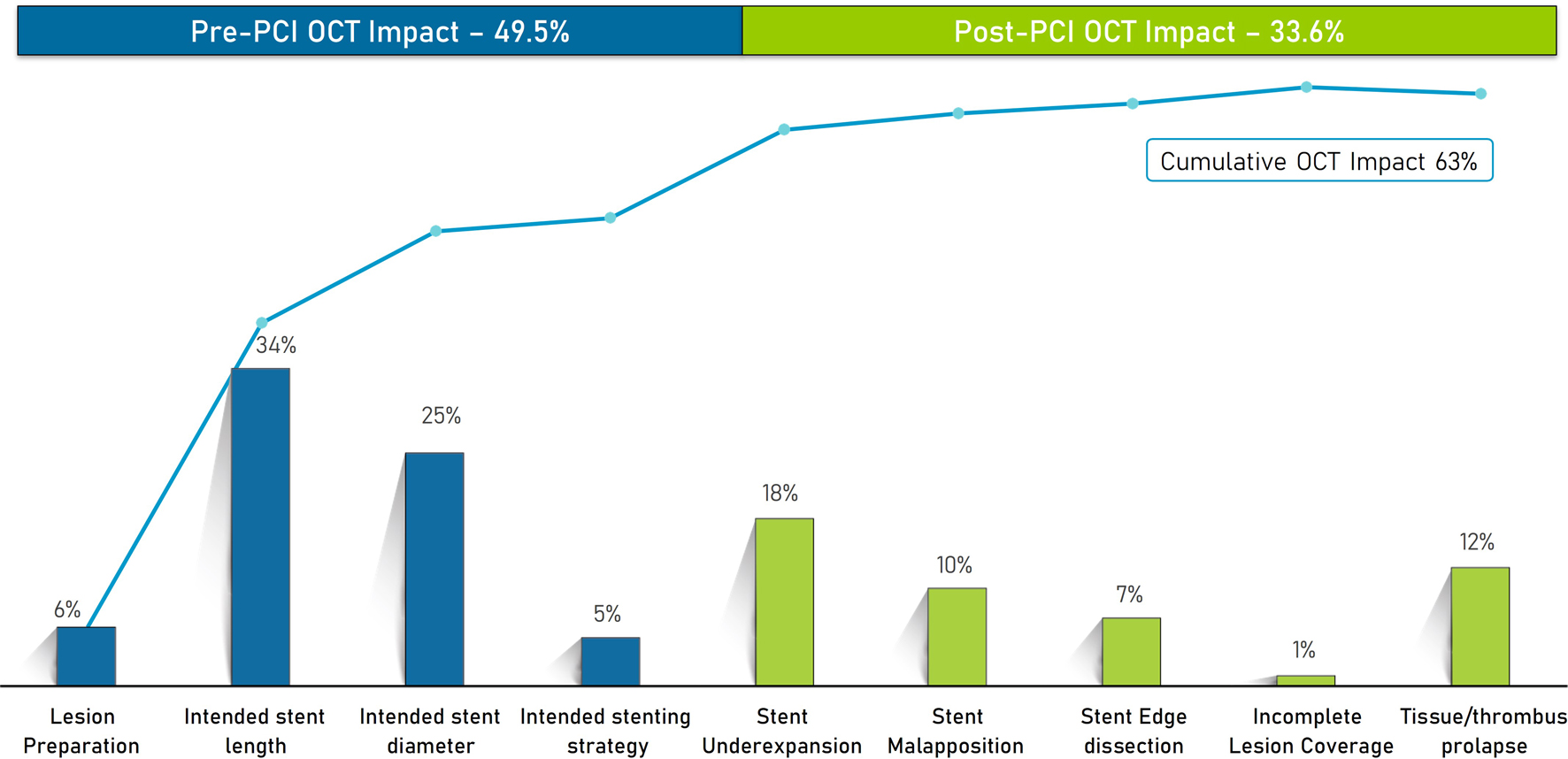 Click for large image | Figure 5. Impact of OCT on pre- and post-PCI strategy. OCT: optical coherence tomography; PCI: percutaneous coronary intervention. |
Primary endpoint
TLF
The overall reported TLF rate was 3.3% (n = 9). Nonfatal MI was observed in five patients during hospitalization, and clinically driven target lesion revascularization (CD-TLR) and cardiac death were noted in two patients each during follow-up.
Secondary endpoints
Stent thrombosis was reported in one patient (0.4%). The rate of concordance between the FFR and RFR values was 91.5%, of which FFRs ≤ 0.80 and RFRs ≤ 0.89 were observed in 26.9% of the lesions and FFRs > 0.80 and RFRs > 0.89 were observed in 64.6% of the lesions. The rate of discordance observed was 8.5%, of which 7.3% of the lesions presented with FFRs > 0.80 and RFRs ≤ 0.89 and 1.2% of the lesions presented with FFRs ≤ 0.80 and RFRs > 0.89 (Fig. 3). Among patients showing concordance with RFR and FFR, one death (0.4%) and CD-TLR in one patient (0.4%) were reported.
The total amount of contrast used for the procedure was 188.4 ± 126.7 mL, which included 44.2 ± 26.1 mL for OCT and 148.1 ± 114.2 mL for angiography. No incidence of contrast-induced nephropathy was noted (pre-PCI serum creatinine: 1.0 ± 0.2 mg/dL, and post-PCI serum creatinine: 1.0 ± 0.4 mg/dL). The time taken for the procedure was 74.6 ± 39.1 min.
| Discussion | ▴Top |
This study is primarily designed to evaluate the feasibility and potential clinical advantages of integrating OCT and FFR in PCI of patients with diabetes. Systematic use of physiology and imaging in diabetic patients resulted in annual TLF rates (3.3%) and stent thrombosis (0.4%).
DM is often associated with complex CAD and additional comorbidities. Acute coronary syndrome constituted 74% in this study, whereas it was 70.2% in a Korean Multicenter Drug-Eluting Stent (DES) registry on diabetic patients [20], 53% in the TUXEDO study [21], comparing paclitaxel- vs. everolimus-eluting stents in patients with diabetes from India [21], and 47.8% in COMBINE OCT-FFR trial [22]. Although 93.4% of coronary lesions had low SYNTAX scores, 68% were categorized as ACC/AHA classification type B and C lesions. Bifurcation lesion was seen in 26.8%, long lesions (≥ 28 mm) in 61.7%, and small vessels (≤ 3 mm) in 61.4%. These features are representative of patients with diabetes who currently undergo PCI in South Asian regions. Among comorbidities, hypertension was the most common (75.9%), followed by dyslipidemia (22.6%). The antidiabetic drugs received by the patients were in accordance with current guideline recommendations. Insulin was prescribed for 40.5% of the patients. The mean duration of diabetes was 80.3 ± 78.5 months.
Guidelines have recommended pressure wire-based physiology assessment (FFR or instantaneous wave-free ratio (iFR)) to indicate the need for PCI among angiographically intermediate coronary lesions [23, 24]. The reliability of intracoronary physiology assessment in patients with diabetes might be interfered with the higher level of microvascular dysfunction and propensity of vulnerable plaques [25]. In the secondary analysis of the DEFINE FLAIR study, physiology-guided PCI was associated with a significantly higher risk of MACEs in patients with diabetes than in nondiabetics (8.6% vs. 5.6%; adjusted hazard ratio: 1.88; 95% CI: 1.28 - 2.64; P < 0.00) [26]. In this study of 353 lesions, 82 have undergone FFR-guided PCI with a deferral rate of 70%. The discordance rate between FFR and RFR was seen only in 8.5% of cases in our study, while previous studies reported a higher prevalence of FFR/non-hyperemic pressure ratios discordance in patients with diabetes [27, 28].
The use of OCT during PCI has been shown to reduce the short and long-term MACE rate, including mortality, TLF, and stent thrombosis, compared to angiography or coronary physiology-guided PCI [13, 29]. Prespecified sub-study analysis of the RENOVATE-COMPLEX-PCI trial [30] showed that ICI guidance reduced the risk of target vessel failure (TVF) compared to angiography guidance in nondiabetic patients but not in patients with diabetes. Diabetes has been observed as a significant predictor of suboptimal stent results and future stent-related cardiac events from pooled data of trials CLI-OPCI II, ILUMIEN-IV OPTIMAL PCI, and FORZA by multivariable analysis [31], emphasizing that stent optimization is highly essential for better outcomes in patients with diabetes. All PCI procedures in this study were guided by OCT using MLD-MAX workflow. A pre-PCI OCT run was performed to define lesion morphology, lesion preparation strategy, stent length, and stent diameter, while a post-PCI OCT run was performed to assess dissections and to optimize stent apposition and expansion. In addition, tissue prolapse, geographical misses, and other complications related to stent placement were detected and corrected. The systematic use of OCT in this study impacted the preprocedural strategy in 49.5% of the lesions and the postprocedural approach in 33.6% of the lesions, leading to an overall cumulative impact on 63% of the lesions. When compared with previous studies, the impact of OCT on the pre-PCI strategy was less pronounced (49.5%) than in the LightLab study (80%) [32] and the iOptico study (86%) [33]. However, the post-PCI impact of OCT was comparable to that reported in the LightLab (31%) and iOptico studies (30%).
Various studies have investigated the TLF rates associated with the use of different stents in patients with CAD and DM. A comprehensive analysis of patients with DM who received Resolute zotarolimus-eluting stent (N = 5,130), comprising data from the RESOLUTE global clinical program, revealed a 12-month TLF rate of 7.8% (upper CI: 9.51%). This rate was significantly lower than the predetermined performance goal of 14.5% (P < 0.001) [17]. Similarly, the EVOLVE II trial (N = 263) reported a 1-year TLF rate of 7.5% [18]. The Korean Multicenter DES registry, which included patients with diabetes undergoing PCI with second-generation DES (N = 1,913), reported a TVR rate of 4.9% at a 2-year follow-up [20]. Additionally, findings from the BASKET-SMALL 2 trial reported a TVR rate of 7.99% at 12 months in DM patients treated with DES [34]. The observed annual TLF rate of 3.3% in the present study was lower than previously reported rates. Our study excluded patients with ST-elevation myocardial infarction, chronic total occlusions, in-stent restenosis, and left main disease, which might have contributed to the observed lower TLF rate. More importantly, the lower rate of MACE could be attributed to the complementary role of physiology guidance of PCI of intermediate lesions, avoiding unnecessary stenting and image guidance of PCI procedures achieving larger final MSA (an average MSA of 6.1 ± 2.1 mm2 in the whole study population, 5.2 ± 1.4 mm2 in small vessels, and 8.2 ± 2.2 mm2 in large vessels) and correction of edge dissection, stent malapposition, and avoidance of geographical miss. In a recent study in Indian patients with complex coronary lesions, OCT-guided PCI has been shown to achieve larger MSA and lower event rates [35]. Furthermore, the strategy of combining physiology and imaging has the potential to achieve effective complete revascularization in a greater number of patients with diabetes.
The total contrast used (188.4 ± 126.7 mL) in this study was lesser than that used in the OCT- angiographic coregistration-guided PCI of the iOptico study (210.0 ± 106.6 mL) [33]. The total procedure time (74.6 ± 39.1 min), which included OCT and physiology assessment, was numerically higher than that in the angiographic arm of the ILUMIEN IV study (mean: 50 ± 35.4 min) [36] and the RENOVATE study (median: 53.5 min) [30].
Limitations
The single-arm design and the absence of a control group (angiography-guided PCI or FFR-only guided PCI) limit the ability to establish causal relationships between the primary endpoint and the use of physiology and imaging in PCI of the study population. Other limitations include the smaller sample size and shorter follow-up duration, which may be insufficient to fully assess the long-term benefits of OCT-guided PCI, as diabetes is associated with cumulative long-term restenosis and cardiovascular events. The absence of a control group is a limitation of this study. However, the results are promising and warrant further investigation through larger, randomized controlled trials to confirm the long-term benefits, assess cost-effectiveness, and provide stronger evidence for the wider use of physiology and imaging in PCI of patients with diabetes.
Conclusion
In patients with diabetes, FFR-based selection of intermediate lesions for PCI and OCT guidance of PCI procedures resulted in a low annual rate of TLF (3.3%). The strategy of systematic combined use of coronary physiology and imaging could lead to improved PCI outcomes in patients with diabetes.
Acknowledgments
The authors would like to thank all study staff (co-investigators and research coordinators) for their support in executing the study, St. Jude Medical India Pvt. Ltd. for the research grant, and BioQuest Solutions Pvt. Ltd. for supporting the execution of the study.
Financial Disclosure
The study has been funded by St. Jude Medical India Pvt. Ltd. (now Abbott). Dr. C. G. Bahuleyan has received research support from St. Jude Medical India Pvt. Ltd. (now Abbott).
Conflict of Interest
All authors declare no relevant financial or non-financial interests to disclose.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author Contributions
Conceptualization: CGB; data curation: CGB, SS, FTNM, SK, MBC, RMK, RK, SR, RA, and AM; formal analysis: CGB and VS; funding acquisition: CGB; investigation: CGB, SS, FTNM, SK, MBC, RMK, RK, SR, RA, and AM; methodology: CGB; project administration: CGB; supervision: CGB; validation: CGB, SS, FTNM, SK, MBC, RMK, RK, SR, RA, AM, and VS; visualization: CGB; writing - original draft: CGB; writing - review and editing: all authors.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ACC/AHA: American College of Cardiology/American Heart Association; BMI: body mass index; CAD: coronary artery disease; CD-TLR: clinically driven target lesion revascularization; CTRI: Clinical Trials Registry - India; D1: diagonal 1; DES: drug-eluting stent; DM: diabetes mellitus; DPP4: dipeptidyl peptidase 4; FFR: fractional flow reserve; FU: follow-up; GLP-1: glucagon-like peptide-1; HRP: high-risk plaque; iFR: instantaneous wave-free ratio; ICI: intracoronary imaging; LAD: left anterior descending; LCX: left circumflex; MACEs: major adverse cardiac events; MAX: Medial dissection, Apposition, eXpansion; MI: myocardial infarction; MLA: minimal lumen area; MLD: Morphology, Length, Diameter; MSA: minimum stent area; NSTEMI: non-ST-segment elevation myocardial infarction; OCT: optical coherence tomography; OM1: obtuse marginal 1; OM2: obtuse marginal 2; OMT: optimal medical therapy; OPTIS™: Optical Coherence Tomography Intravascular Imaging System; PCI: percutaneous coronary intervention; PDA: posterior descending artery; PLV: posterior left ventricular; RCA: right coronary artery; RFR: resting full-cycle ratio; RI: ramus intermedius; RVD: reference vessel diameter; SD: standard deviation; SGLT-2: sodium-glucose co-transporter 2; SIHD: stable ischemic heart disease; STEMI: ST-elevation myocardial infarction; SYNTAX: Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery; TLF: target lesion failure; TVF: target vessel failure; TVR: target vessel revascularization; UA: unstable angina
| References | ▴Top |
- Hills AP, Arena R, Khunti K, Yajnik CS, Jayawardena R, Henry CJ, Street SJ, et al. Epidemiology and determinants of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018;6(12):966-978.
doi pubmed - Ledru F, Ducimetiere P, Battaglia S, Courbon D, Beverelli F, Guize L, Guermonprez JL, et al. New diagnostic criteria for diabetes and coronary artery disease: insights from an angiographic study. J Am Coll Cardiol. 2001;37(6):1543-1550.
doi pubmed - Niccoli G, Giubilato S, Di Vito L, Leo A, Cosentino N, Pitocco D, Marco V, et al. Severity of coronary atherosclerosis in patients with a first acute coronary event: a diabetes paradox. Eur Heart J. 2013;34(10):729-741.
doi pubmed - Nogic J, Nerlekar N, Soon K, Freeman M, Chan J, Roberts L, Brenan A, et al. Diabetes mellitus is independently associated with early stent thrombosis in patients undergoing drug eluting stent implantation: Analysis from the Victorian cardiac outcomes registry. Catheter Cardiovasc Interv. 2022;99(3):554-562.
doi pubmed - Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van' t Veer M, Klauss V, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213-224.
doi pubmed - Parikh RV, Liu G, Plomondon ME, Sehested TSG, Hlatky MA, Waldo SW, Fearon WF. Utilization and outcomes of measuring fractional flow reserve in patients with stable ischemic heart disease. J Am Coll Cardiol. 2020;75(4):409-419.
doi pubmed - Sud M, Han L, Koh M, Austin PC, Farkouh ME, Ly HQ, Madan M, et al. Association between adherence to fractional flow reserve treatment thresholds and major adverse cardiac events in patients with coronary artery disease. JAMA. 2020;324(23):2406-2414.
doi pubmed - Sanz Sanchez J, Farjat Pasos JI, Martinez Sole J, Hussain B, Kumar S, Garg M, Chiarito M, et al. Fractional flow reserve use in coronary artery revascularization: A systematic review and meta-analysis. iScience. 2023;26(8):107245.
doi pubmed - Kennedy MW, Kaplan E, Hermanides RS, Fabris E, Hemradj V, Koopmans PC, Dambrink JH, et al. Clinical outcomes of deferred revascularisation using fractional flow reserve in patients with and without diabetes mellitus. Cardiovasc Diabetol. 2016;15:100.
doi pubmed - Scarsini R, Tebaldi M, Rubino F, Sgreva S, Vescovo G, Barbierato M, Vicere A, et al. Intracoronary physiology-guided percutaneous coronary intervention in patients with diabetes. Clin Res Cardiol. 2023;112(9):1331-1342.
doi pubmed - Khan SU, Agarwal S, Arshad HB, Akbar UA, Mamas MA, Arora S, Baber U, et al. Intravascular imaging guided versus coronary angiography guided percutaneous coronary intervention: systematic review and meta-analysis. BMJ. 2023;383:e077848.
doi pubmed - Yasmin F, Jawed K, Moeed A, Ali SH. Efficacy of intravascular imaging-guided drug-eluting stent implantation: a systematic review and meta-analysis of randomized clinical trials. Curr Probl Cardiol. 2024;49(1 Pt A):102002.
doi pubmed - Bergmark BA, Osborn EA, Ali ZA, Gupta A, Kolli KK, Prillinger JB, Hasegawa J, et al. Association between intracoronary imaging during pci and clinical outcomes in a real-world US Medicare population. J Soc Cardiovasc Angiogr Interv. 2023;2(2):100556.
doi pubmed - Stone GW, Christiansen EH, Ali ZA, Andreasen LN, Maehara A, Ahmad Y, Landmesser U, et al. Intravascular imaging-guided versus angiography-guided PCI: systematic review and meta-analysis of 24 randomized controlled trials. medRxiv. 2023.
- Shlofmitz E, Croce K, Bezerra H, Sheth T, Chehab B, West NEJ, Shlofmitz R, et al. The MLD MAX OCT algorithm: An imaging-based workflow for percutaneous coronary intervention. Catheter Cardiovasc Interv. 2022;100(Suppl 1):S7-S13.
doi pubmed - Konigstein M, Redfors B, Zhang Z, Kotinkaduwa LN, Mintz GS, Smits PC, Serruys PW, et al. Utility of the ACC/AHA lesion classification to predict outcomes after contemporary DES treatment: individual patient data pooled analysis from 7 randomized trials. J Am Heart Assoc. 2022;11(24):e025275.
doi pubmed - Silber S, Serruys PW, Leon MB, Meredith IT, Windecker S, Neumann FJ, Belardi J, et al. Clinical outcome of patients with and without diabetes mellitus after percutaneous coronary intervention with the resolute zotarolimus-eluting stent: 2-year results from the prospectively pooled analysis of the international global RESOLUTE program. JACC Cardiovasc Interv. 2013;6(4):357-368.
doi pubmed - Kereiakes DJ, Meredith IT, Masotti M, Carrie D, Moreno R, Erglis A, Mehta SR, et al. Safety and efficacy of a bioabsorbable polymer-coated, everolimus-eluting coronary stent in patients with diabetes: the EVOLVE II diabetes substudy. EuroIntervention. 2017;12(16):1987-1994.
doi pubmed - Food and Drug Administration. Guidelines for Industry: Non-Inferiority Clinical Trials. March 2010. Available at: http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm202140.pdf. Accessed December, 26 2023.
- Lee CH, Choi SW, Jun SW, Hwang J, Kim IC, Cho YK, Park HS, et al. Clinical impact of diabetes mellitus on 2-year clinical outcomes following PCI with second-generation drug-eluting stents; Landmark analysis findings from patient registry: Pooled analysis of the Korean multicenter drug-eluting stent registry. PLoS One. 2020;15(6):e0234362.
doi pubmed - Kaul U, Bangalore S, Seth A, Arambam P, Abhaichand RK, Patel TM, Banker D, et al. Paclitaxel-eluting versus everolimus-eluting coronary stents in diabetes. N Engl J Med. 2015;373(18):1709-1719.
doi pubmed - Kedhi E, Berta B, Roleder T, Hermanides RS, Fabris E, AJJ IJ, Kauer F, et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: the COMBINE OCT-FFR trial. Eur Heart J. 2021;42(45):4671-4679.
doi pubmed - Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87-165.
doi pubmed - Escaned J, Berry C, De Bruyne B, Shabbir A, Collet C, Lee JM, Appelman Y, et al. Applied coronary physiology for planning and guidance of percutaneous coronary interventions. A clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) of the European Society of Cardiology. EuroIntervention. 2023;19(6):464-481.
doi pubmed - Leung M, Leung DY. Coronary microvascular function in patients with type 2 diabetes mellitus. EuroIntervention. 2016;11(10):1111-1117.
doi pubmed - Investigators D-FT, Lee JM, Choi KH, Koo BK, Dehbi HM, Doh JH, Nam CW, et al. Comparison of major adverse cardiac events between instantaneous wave-free ratio and fractional flow reserve-guided strategy in patients with or without type 2 diabetes: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 2019;4(9):857-864.
doi pubmed - Lee JM, Shin ES, Nam CW, Doh JH, Hwang D, Park J, Kim KJ, et al. Discrepancy between fractional flow reserve and instantaneous wave-free ratio: Clinical and angiographic characteristics. Int J Cardiol. 2017;245:63-68.
doi pubmed - Yamazaki T, Saito Y, Kobayashi T, Kitahara H, Kobayashi Y. Factors associated with discordance between fractional flow reserve and resting full-cycle ratio. J Cardiol. 2022;80(1):9-13.
doi pubmed - Kuno T, Kiyohara Y, Maehara A, Ueyama HA, Kampaktsis PN, Takagi H, Mehran R, et al. Comparison of intravascular imaging, functional, or angiographically guided coronary intervention. J Am Coll Cardiol. 2023;82(23):2167-2176.
doi pubmed - Lee JM, Choi KH, Song YB, Lee JY, Lee SJ, Lee SY, Kim SM, et al. Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N Engl J Med. 2023;388(18):1668-1679.
doi pubmed - Romagnoli E, Burzotta F, Vergallo R, Gatto L, Biondi-Zoccai G, Ramazzotti V, Biccire F, et al. Clinical impact of OCT-derived suboptimal stent implantation parameters and definitions. Eur Heart J Cardiovasc Imaging. 2023;25(1):48-57.
doi pubmed - Bergmark B, Dallan LAP, Pereira GTR, Kuder JF, Murphy SA, Buccola J, Wollmuth J, et al. Decision-making during percutaneous coronary intervention guided by optical coherence tomography: insights from the LightLab initiative. Circ Cardiovasc Interv. 2022;15(11):872-881.
doi pubmed - Kadavil RM, Abdullakutty J, Patel T, Rathnavel S, Singh B, Chouhan NS, Malik FTN, et al. Impact of real-time optical coherence tomography and angiographic coregistration on the percutaneous coronary intervention strategy. AsiaIntervention. 2023;9(2):124-132.
doi pubmed - Wohrle J, Scheller B, Seeger J, Farah A, Ohlow MA, Mangner N, Mobius-Winkler S, et al. Impact of diabetes on outcome with drug-coated balloons versus drug-eluting stents: the BASKET-SMALL 2 trial. JACC Cardiovasc Interv. 2021;14(16):1789-1798.
doi pubmed - Chandra P, Sethuraman S, Roy S, Mohanty A, Parikh K, Charantharalyil Gopalan B, Sahoo PK, et al. Effectiveness and safety of optical coherence tomography-guided PCI in Indian patients with complex lesions: A multicenter, prospective registry. Indian Heart J. 2023;75(4):236-242.
doi pubmed - Ali ZA, Landmesser U, Maehara A, Matsumura M, Shlofmitz RA, Guagliumi G, Price MJ, et al. Optical coherence tomography-guided versus angiography-guided PCI. N Engl J Med. 2023;389(16):1466-1476.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.