| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 000, Number 000, April 2025, pages 000-000
Lipid-Lowering Therapy in Post-Acute Coronary Syndrome Patients: An Observational Study
Meshari S. Alwagdania, c, Naeem Alshoaibib, Hossameldeen M. Elghetanya, Abdulrahman H. Algrigria, Razan A. Bahurmuza, Abdulwahab A. Alqahtania, Zeyad T. Olfata, Hatun Halawania
aDepartment of Cardiology, Doctor Soliman Fakeeh Hospital, Jeddah 23434, Saudi Arabia
bDepartment of Cardiology, King Abdulaziz University Hospital, Jeddah 22252, Saudi Arabia
cCorresponding Author: Meshari S. Alwagdani, Department of Cardiology, Doctor Soliman Fakeeh Hospital, PO Box 9201, Jeddah 23434, Saudi Arabia
Manuscript submitted March 17, 2025, accepted April 12, 2025, published online April 22, 2025
Short title: LLT in Post-ACS Patients
doi: https://doi.org/10.14740/cr2063
| Abstract | ▴Top |
Background: Cardiovascular disease remains a major cause of morbidity and mortality globally. International guidelines recommend aggressive lipid-lowering therapy (LLT) in patients with atherosclerotic cardiovascular disease (ASCVD), targeting a low-density lipoprotein cholesterol (LDL-C) level of < 55 mg/dL and a ≥ 50% reduction from baseline. However, real-world studies continue to show suboptimal LDL-C target achievement. This study aimed to assess the proportion of post-acute coronary syndrome (ACS) patients achieving both LDL-C < 55 mg/dL and a ≥ 50% reduction from baseline at 6 months. A secondary objective was to evaluate target achievement after 1 year and analyze outcomes across different LLT regimens.
Methods: We conducted a retrospective cohort study at a single tertiary center, including patients aged ≥ 18 years who presented with ACS between January 2021 and January 2022, underwent percutaneous coronary intervention (PCI), and had documented LDL-C levels at baseline and at least one follow-up within 12 months. Patients with baseline LDL-C ≤ 55 mg/dL or on ongoing LLT were excluded.
Results: A total of 122 patients were included (mean age 63.5 years; 59.8% had both diabetes and hypertension). At 6 months, only 13/82 patients (15.9%) achieved the primary LDL-C target. The highest achievement was seen in the rosuvastatin + ezetimibe group (30.0%), followed by rosuvastatin (17.9%), atorvastatin + ezetimibe (14.3%), and atorvastatin monotherapy (14.0%). A ≥ 50% LDL-C reduction without meeting the < 55 mg/dL threshold was observed in 24/82 patients (29.3%).
Conclusions: LDL-C target achievement remains low among post-ACS patients despite high-intensity statin use. Combination therapy with rosuvastatin + ezetimibe showed more favorable outcomes, particularly in older adults. These findings underscore the need for structured follow-up, treatment intensification, and broader use of advanced therapies such as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors to close the real-world treatment gap.
Keywords: Acute coronary syndrome; Lipid-lowering therapy; Atherosclerosis; LDL-C; Cardiovascular disease
| Introduction | ▴Top |
Cardiovascular diseases (CVDs) continue to be the leading cause of morbidity and mortality globally, accounting for an estimated 17.9 million deaths per year [1-3]. Among the key modifiable risk factors, dyslipidemia, particularly elevated levels of low-density lipoprotein cholesterol (LDL-C), plays a central role in the pathogenesis of atherosclerosis and its clinical manifestations. In Saudi Arabia, dyslipidemia has been reported in up to 68.8% of the population, making it the most prevalent cardiovascular risk factor in the region [4].
The clinical benefits of lipid-lowering therapy (LLT), particularly statins, in reducing major cardiovascular events have been well documented across randomized trials and large observational cohorts [5]. Accordingly, international guidelines from both the American College of Cardiology (ACC) and the European Society of Cardiology (ESC) recommend initiating or intensifying LLT in patients with established atherosclerotic cardiovascular disease (ASCVD) to achieve stringent LDL-C targets. The ESC recommends reducing LDL-C to < 55 mg/dL, while the ACC recommends a target of < 70 mg/dL in high-risk populations. Both societies emphasize the importance of achieving at least a 50% reduction from baseline LDL-C levels [6, 7]. This was later on reiterated by Virani et al in their 2023 guideline for the management of patients with chronic coronary diseases [8].
Despite the availability of potent LLT options, including high-intensity statins, ezetimibe, and proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9i), real-world data consistently show a substantial gap between guideline-recommended targets and clinical practice. Observational studies from various regions, including the Middle East, have demonstrated that a large proportion of patients fail to achieve LDL-C goals, often due to therapeutic inertia, poor follow-up, or underuse of combination therapy [5, 9]. PCSK9 inhibitors, although effective in achieving deeper LDL-C reductions and improving adherence [10], remain underutilized in many health systems, partly due to cost, limited access, and prescribing hesitancy [5, 9].
This study was conducted to evaluate real-world LDL-C control in a cohort of patients who presented with acute coronary syndrome (ACS) and underwent percutaneous coronary intervention (PCI) at a single tertiary center. Specifically, we aimed to assess the proportion of patients achieving both a ≥ 50% reduction in LDL-C and an absolute LDL-C level < 55 mg/dL at two key follow-up intervals: 6 months and 1-year post-discharge. In addition, we examined the distribution and effectiveness of different LLT regimens, including combination therapies and the use of PCSK9 inhibitors, to understand treatment patterns and their impact on LDL-C target achievement.
| Materials and Methods | ▴Top |
This retrospective observational study was conducted at Dr. Soliman Fakeeh Hospital, a tertiary care center in Jeddah, Saudi Arabia. The hospital’s electronic medical records were reviewed to identify patients who presented with ACS between January 2021 and January 2022. Patients were screened using the coronary angiography procedure code.
To be eligible for inclusion, patients had to meet the following criteria: 1) aged 18 years or older; 2) presented with ACS and underwent percutaneous coronary angiography; 3) initiated on LLT at the time of presentation; and 4) had documented LDL-C levels at baseline and at least one follow-up measurement within the subsequent 12 months. Patients with a baseline LDL-C level of ≤ 55 mg/dL or those already on LLT prior to admission were excluded from the study.
A total of 122 patients met these criteria and were included in the analysis. Patients were classified into five treatment groups based on the LLT regimen prescribed at discharge: 1) rosuvastatin monotherapy; 2) atorvastatin monotherapy; 3) rosuvastatin + ezetimibe; 4) atorvastatin + ezetimibe; and 5) rosuvastatin + PCSK9 inhibitor. All patients were initiated on high-intensity statin therapy in accordance with international guidelines. Specifically, patients in the rosuvastatin group received 20 mg or 40 mg daily, and those in the atorvastatin group received 40 mg or 80 mg daily. For those receiving combination therapy, ezetimibe 10 mg daily was added to the statin regimen. One patient received evolocumab 140 mg subcutaneously every 2 weeks alongside rosuvastatin 40 mg daily. Dosage selection was based on physician discretion, considering individual patient risk profiles and clinical judgment.
Patients were assigned to treatment groups based on their initial discharge regimen. If ezetimibe or other lipid-lowering agents were added during follow-up, the patient remained in their original treatment group to preserve consistency in analysis. This classification approach was chosen to reflect the intended discharge strategy, as medication changes post-discharge were not consistently documented.
LDL-C follow-up measurements were collected opportunistically during routine outpatient visits, as there was no standardized follow-up protocol. Among the 122 patients, 29 (23.8%) had LDL-C measured at approximately 6 weeks, 82 (67.2%) at or near 6 months, and 74 (60.7%) at approximately 1 year. Patients without at least one follow-up LDL-C value after baseline were excluded from outcome analysis. For those included, LDL-C data were analyzed at one or more of the following time points: 6 weeks, 6 months, and 1 year. Each treatment group was further stratified based on the timing of available LDL-C measurements.
Although this study shares characteristics with a clinical audit, such as evaluating adherence to established LDL-C treatment goals, the primary aim was to generate real-world evidence on the effectiveness of LLT regimens in post-ACS patients. Rather than focusing solely on institutional benchmarking, this study aimed to explore treatment responses and LDL-C outcome trends that may inform future secondary prevention strategies.
The main objective of the study was to evaluate the proportion of patients who achieved both a ≥ 50% reduction in LDL-C from baseline and an absolute LDL-C level < 55 mg/dL. The primary assessment was conducted at the 6-month follow-up, given that this time point had the highest rate of available data. The primary hypothesis was that a significant proportion of patients would not achieve the LDL-C target despite initiation of high-intensity LLT. Descriptive statistics, subgroup analysis, and Chi-square testing were used to evaluate target achievement across treatment groups. Although the study was not powered for formal hypothesis testing, subgroup comparisons and P-values were reported to identify possible trends and clinically meaningful differences.
The secondary objective was to assess the same composite outcome at 1 year. Additionally, the proportion of patients achieving either a ≥ 50% LDL-C reduction or an LDL-C < 55 mg/dL at both 6 months and 1 year was analyzed to provide further context on partial target achievement.
Statistical analysis
All data were analyzed using IBM SPSS version 27 (IBM Corp., Armonk, NY, USA). Descriptive statistics were used to summarize baseline characteristics. Continuous variables, such as age and LDL-C levels at baseline and follow-up time points, were expressed as mean ± standard deviation. Categorical variables, such as gender, comorbidities, and LLT regimens, were presented as counts and percentages. To evaluate associations between LLT regimens and the achievement of the primary outcome (LDL-C < 55 mg/dL and ≥ 50% reduction from baseline at 6 months), we used the Chi-square test. Additional subgroup analyses were performed based on age group, gender, and comorbidity status (diabetes, hypertension, or both). A P-value of < 0.05 was considered statistically significant. Due to the observational design and small sample sizes within subgroups, these comparisons were exploratory and intended to support hypothesis generation rather than infer causality. One statistically significant finding was observed in the rosuvastatin + ezetimibe group within the 61 - 70 age category (P = 0.032), suggesting a possible association in this subgroup. Other group comparisons did not reach statistical significance but are reported for clinical context.
| Results | ▴Top |
Patient characteristics
A total of 122 patients were included in the analysis. The mean age was 63.5 ± 10.9 years, and 90 (73.8%) were male. Regarding comorbidities, 73 patients (59.8%) had both diabetes and hypertension, 15 (12.3%) had diabetes alone, 23 (18.9%) had hypertension alone, and 11 (9.0%) had neither condition (Table 1). The mean baseline LDL-C was 117.2 ± 41.3 mg/dL.
 Click to view | Table 1. Baseline Demographic and Clinical Characteristics of the Study Population (N = 122) |
LLT use
At discharge, 39 patients (32.0%) were started on rosuvastatin monotherapy, 25 (20.5%) on atorvastatin monotherapy, 43 (35.2%) on atorvastatin + ezetimibe, 14 (11.5%) on rosuvastatin + ezetimibe, and one patient (0.8%) received a PCSK9 inhibitor (evolocumab) in addition to rosuvastatin (Table 2).
 Click to view | Table 2. Frequency and Distribution of Lipid-Lowering Therapies Prescribed at Discharge |
LDL-C measurements
Follow-up LDL-C measurements were available for 29 patients (23.8%) at approximately 6 weeks, 82 patients (67.2%) at 6 months, and 74 patients (60.7%) at 1 year (Table 3). At 6 months, the mean LDL-C was 84.5 ± 39.6 mg/dL. At 1 year, it was 83.7 ± 43.7 mg/dL.
 Click to view | Table 3. LDL-C Levels at Baseline and Follow-Up Time Points, and Primary Outcome Achievement at 6 Months |
Primary outcome achievement at 6 months
Among the 82 patients with 6-month LDL-C follow-up data, 13 (15.9%) met the primary outcome: LDL-C < 55 mg/dL with ≥ 50% reduction from baseline (Table 3). Outcome rates by treatment group were as follows (Table 4; Fig. 1): 1) rosuvastatin monotherapy: 7/39 (17.9%); 2) atorvastatin monotherapy: 6/43 (14.0%); 3) ezetimibe (overall): 7/38 (18.4%); 4) atorvastatin + ezetimibe: 4/28 (14.3%); 5) rosuvastatin + ezetimibe: 3/10 (30.0%); and 6) PCSK9i: 0/1 (0%), the patient did not achieve the primary outcome. While these differences were not statistically significant (all P > 0.2), the numerically higher rate in the rosuvastatin + ezetimibe group may reflect a potential clinical trend. The small sample size in each group likely contributed to limited statistical power.
 Click to view | Table 4. Achievement of Primary LDL-C Target at 6 Months by Treatment Group (LDL-C < 55 mg/dL and ≥ 50% Reduction) |
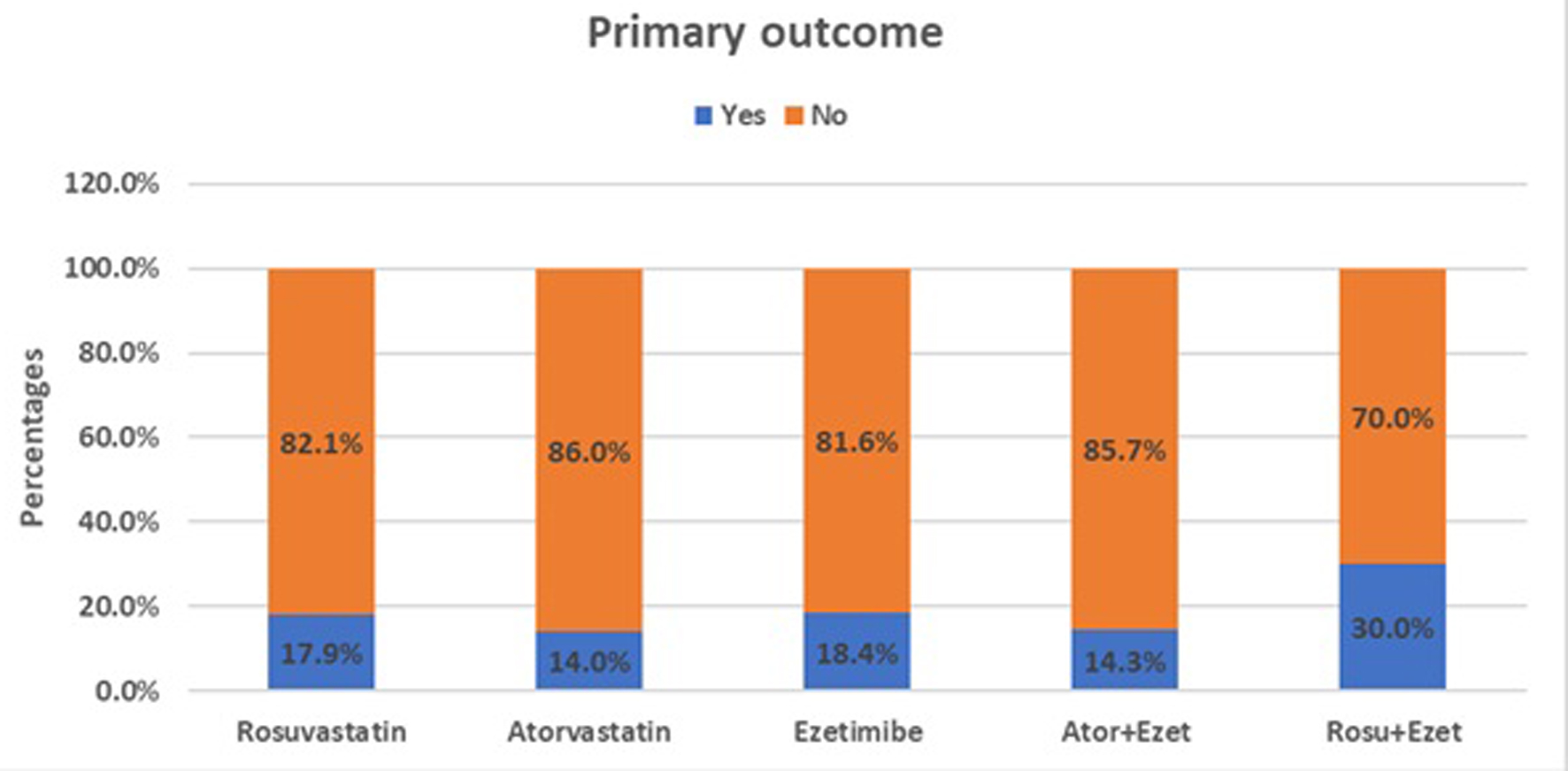 Click for large image | Figure 1. Proportion of patients achieving the primary LDL-C target (LDL-C < 55 mg/dL and ≥ 50% reduction from baseline) across lipid-lowering therapy groups at 6 months. LDL-C: low-density lipoprotein-cholesterol. |
Subgroup analyses
Age
In patients aged 61 - 70 years receiving rosuvastatin + ezetimibe, two of four patients (50%) achieved the primary LDL-C target (P = 0.032). No other age-stratified subgroup showed significant differences (Table 5).
 Click to view | Table 5. Primary LDL-C Target Achievement at 6 Months Stratified by Age Group and Treatment Regimen |
Gender
Across all regimens, LDL-C target achievement rates were similar between males and females, for instance: 1) in the rosuvastatin monotherapy group: 6/30 (20.0%) of males vs. 1/9 (11.1%) of females; and 2) in the atorvastatin group: 5/31 (16.1%) of males vs. 1/12 (8.3%) of females. None of these differences reached statistical significance (all P > 0.1) (Table 6).
 Click to view | Table 6. Primary LDL-C Target Achievement at 6 Months Stratified by Gender and Treatment Regimen |
Comorbidities
When stratified by comorbidity status, no statistically significant association with LDL-C outcome was observed, for example: 1) rosuvastatin group: 0/2 (0%) of patients with diabetes alone, 5/23 (21.7%) with both diabetes and hypertension; and 2) atorvastatin group: 3/10 (30.0%) with diabetes alone, 2/22 (9.1%) with both. Small subgroup sizes and the retrospective design limit the interpretation of these trends (Table 7).
 Click to view | Table 7. Primary LDL-C Target Achievement at 6 Months Stratified by Comorbidity Profile and Treatment Regimen |
Secondary outcomes at 1 year
Among the 74 patients with LDL-C data at 1 year, 11 (14.9%) met the composite outcome (LDL-C < 55 mg/dL and ≥ 50% reduction from baseline). Group-specific rates were: 1) rosuvastatin monotherapy: 5/25 (20.0%); 2) rosuvastatin + ezetimibe: 2/10 (20.0%); 3) atorvastatin + ezetimibe: 3/24 (12.5%); 4) ezetimibe (overall): 5/34 (14.7%); 5) atorvastatin monotherapy: 0/14 (0%); and 6) PCSK9i: one patient achieved ≥ 50% reduction but not LDL-C < 55 mg/dL, thus not meeting the full composite outcome.
≥ 50% LDL-C reduction at 6 months and 1 year
At 6 months, 24 of 82 patients (27.9%) achieved ≥ 50% LDL-C reduction: rosuvastatin + ezetimibe: 6/10 (60.0%); ezetimibe (overall): 14/38 (36.8%); atorvastatin + ezetimibe: 8/28 (28.6%); atorvastatin monotherapy: 4/15 (26.7%); rosuvastatin monotherapy: 6/28 (21.4%); PCSK9i: 0/1 (0%).
At 1 year, 24 of 74 patients (32.4%) achieved ≥ 50% LDL-C reduction: rosuvastatin monotherapy: 12/25 (48.0%); atorvastatin + ezetimibe: 6/24 (25.0%); ezetimibe (overall): 8/34 (23.5%); atorvastatin monotherapy: 3/14 (21.4%); rosuvastatin + ezetimibe: 2/10 (20.0%); PCSK9i: 1/1 (100%), the patient achieved ≥ 50% reduction, but not the composite target.
| Discussion | ▴Top |
In this retrospective observational study conducted at a tertiary care center, we evaluated LDL-C target achievement among post-ACS patients initiated on high-intensity LLT. Despite the use of guideline-recommended regimens, the results highlight persistent gaps in achieving optimal LDL-C levels in real-world clinical practice.
At 6 months, only 13 out of 82 patients (15.9%) achieved the composite LDL-C target of < 55 mg/dL with a ≥ 50% reduction from baseline. This finding is consistent with earlier reports, including the DA VINCI and CEPHEUS studies, which have shown that even among high-risk populations, a large proportion of patients fall short of guideline-directed lipid targets [5, 11]. While combination therapy yielded numerically higher rates of LDL-C goal attainment, these differences did not reach statistical significance, most likely due to the limited number of patients in each subgroup.
Among the various treatment regimens, the rosuvastatin + ezetimibe group achieved the highest composite success rate at 6 months (3/10; 30.0%), followed by the overall ezetimibe cohort (7/38; 18.4%). Lower rates were observed with atorvastatin monotherapy (6/43; 14.0%) and atorvastatin + ezetimibe (4/28; 14.3%). Although the between-group differences were not statistically significant (P > 0.2), the observed trend is consistent with prior studies indicating that the addition of ezetimibe improves LDL-C lowering beyond statin monotherapy [9, 10].
The subgroup analysis revealed a statistically significant finding in patients aged 61 - 70 years receiving rosuvastatin + ezetimibe, where 50% (2/4) achieved the LDL-C target (P = 0.032). No significant associations were observed based on gender or comorbidity profiles, though numerical trends favored males and patients without comorbidities. These findings align with prior evidence suggesting that older adults may benefit more from intensified LLT strategies [10].
Regarding LDL-C reduction alone (≥ 50% from baseline), 27.9% (24/82) achieved this at 6 months, and 32.4% (24/74) at 1 year. The highest 6-month response was observed in the rosuvastatin + ezetimibe group (6/10; 60%), while at 1 year, rosuvastatin monotherapy had the highest rate (12/25; 48%). This sustained performance may reflect better long-term adherence or intrinsic pharmacological durability.
The lone patient on PCSK9 inhibitor therapy achieved a ≥ 50% LDL-C reduction at 1 year but did not meet the composite LDL-C target at 6 months. This underutilization of PCSK9 inhibitors, despite their proven efficacy in high-risk populations, mirrors findings from registries like AT-TARGET-IT, where access and prescribing patterns remain limited [5].
The decline in composite goal achievement from 15.9% at 6 months to 14.9% at 1 year could be attributed to lack of treatment escalation, poor follow-up adherence, or medication non-compliance over time. Even though 35.2% (43/122) of patients were prescribed combination therapy, overall LDL-C control remained suboptimal, suggesting that initiation alone is not enough: sustained monitoring and intensification are essential.
It is also important to acknowledge that this study shares certain characteristics with a clinical audit, particularly in evaluating adherence to LDL-C targets. However, our primary aim was to generate real-world evidence on the effectiveness of LLT regimens in a post-ACS population. Unlike a standard audit, we performed stratified subgroup analysis and formal statistical comparisons between treatment strategies, with the intent to generate insights that could inform prospective research and guide clinical decision-making.
The broader underperformance in LDL-C control likely reflects system-level barriers such as therapeutic inertia, underuse of potent agents, limited access to specialized lipid services, and fragmented follow-up care. In our cohort, follow-up LDL-C testing was not standardized and occurred opportunistically, with only 67.2% (n = 82) and 60.7% (n = 74) of patients having values at 6 months and 1 year, respectively. This variability reduces the likelihood of timely therapeutic adjustments and may have impacted outcome trends.
Taken together, these findings underscore the need for structured lipid management protocols post-ACS. Interventions such as dedicated lipid clinics, routine risk reassessment, timely LDL-C monitoring, and broader access to combination or advanced therapies (e.g., PCSK9 inhibitors) may be key in closing the gap between guideline recommendations and real-world outcomes [10, 11].
Conclusion
In this real-world observational study of post-ACS patients initiated on high-intensity LLT, we found that LDL-C target achievement remains limited despite adherence to guideline-recommended regimens. At 6 months, only 15.9% of patients achieved both an LDL-C level < 55 mg/dL and a ≥ 50% reduction from baseline, with modest improvements seen at 1 year. Combination therapy with rosuvastatin and ezetimibe showed numerically higher success rates, particularly in older adults, but statistical significance was limited by small sample sizes.
These findings highlight the persistent gap between clinical guidelines and practice, likely influenced by suboptimal follow-up, underuse of PCSK9 inhibitors, and lack of treatment intensification. To improve secondary prevention outcomes in high-risk patients, there is a clear need for structured follow-up protocols, timely lipid profile monitoring, and more aggressive, individualized treatment approaches.
Future studies with larger cohorts and prospective designs are warranted to further assess the long-term effectiveness of different LLT strategies and to identify practical solutions for improving LDL-C control in real-world settings.
Limitations
This study has several important limitations. First, its retrospective and observational design inherently limits the ability to draw causal inferences between treatment regimens and LDL-C outcomes. Although subgroup comparisons were made, the results should be interpreted as exploratory due to the lack of randomization and potential confounders that could not be adjusted for statistically.
Second, the study relied on opportunistic LDL-C follow-up data collected during routine clinical visits. There was no standardized follow-up protocol, and some patients may have undergone follow-up testing elsewhere or not returned for lipid monitoring. This introduces the possibility of selection bias, as patients who returned for follow-up may differ systematically from those who did not. Consequently, the true treatment effect may be under- or overestimated.
Third, the sample size was relatively small, particularly within individual treatment subgroups. This limited the statistical power to detect significant differences between regimens and restricted the ability to perform multivariable adjustments for comorbidities or baseline characteristics. As such, any observed trends must be interpreted cautiously and viewed as hypothesis-generating.
Fourth, while the study examined lipid-lowering efficacy, clinical outcomes such as recurrent ACS, revascularization, or cardiovascular mortality were not assessed. Additionally, data on treatment adherence, adverse events (e.g., statin-associated muscle symptoms), and medication adjustments post-discharge were not available.
Finally, patients were categorized based on their discharge LLT regimen, and any subsequent changes in therapy (e.g., ezetimibe or PCSK9i initiation) were not used to reclassify patients. This may not fully capture the dynamic nature of real-world lipid management but was necessary to preserve consistency in analysis and group comparison.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
MSA: conceptualization, methodology, analysis and interpretation of data, investigation, resources, data collection, writing-original draft, writing-review and editing of the final draft of manuscript, visualization, supervision and project administration. NA: conceptualization, methodology, writing-review and editing of the final draft of manuscript, supervision and project administration. HME: conceptualization, methodology, writing-review and editing of the final draft of manuscript, supervision and project administration. AHA, RAB, AAA, ZTO, and HH: investigation, data collection and writing-review and editing of the final draft of manuscript. All authors reviewed the final version of the manuscript and approves it for publication.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ACS: acute coronary syndrome; ASCVD: atherosclerotic cardiovascular diseases; LDL-C: low-density lipoprotein-cholesterol; LLT: lipid-lowering therapy; PCSK9i: proprotein convertase subtilisin/kexin type 9 inhibitors
| References | ▴Top |
- Mortality G.B.D. Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117-171.
doi pubmed - Laslett LJ, Alagona P, Jr., Clark BA, 3rd, Drozda JP, Jr., Saldivar F, Wilson SR, Poe C, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(25 Suppl):S1-49.
doi pubmed - Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, Murray CJ. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132(17):1667-1678.
doi pubmed - Ahmed AM, Hersi A, Mashhoud W, Arafah MR, Abreu PC, Al Rowaily MA, Al-Mallah MH. Cardiovascular risk factors burden in Saudi Arabia: The Africa Middle East Cardiovascular Epidemiological (ACE) study. J Saudi Heart Assoc. 2017;29(4):235-243.
doi pubmed - Cannon CP, de Lemos JA, Rosenson RS, Ballantyne CM, Liu Y, Gao Q, Palagashvilli T, et al. Use of lipid-lowering therapies over 2 years in GOULD, a registry of patients with atherosclerotic cardiovascular disease in the US. JAMA Cardiol. 2021;6(9):1060-1068.
doi pubmed - Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(24):e285-e350.
doi pubmed - Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188.
doi pubmed - Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, Dixon DL, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023;148(9):e9-e119.
doi pubmed - Vrablik M, Seifert B, Parkhomenko A, Banach M, Jozwiak JJ, Kiss RG, Gaita D, et al. Lipid-lowering therapy use in primary and secondary care in Central and Eastern Europe: DA VINCI observational study. Atherosclerosis. 2021;334:66-75.
doi pubmed - Gargiulo P, Basile C, Cesaro A, Marzano F, Buonocore D, Asile G, Abbate V, et al. Efficacy, safety, adherence and persistence of PCSK9 inhibitors in clinical practice: A single country, multicenter, observational study (AT-TARGET-IT). Atherosclerosis. 2023;366:32-39.
doi pubmed - Al Rasadi KH, Al-Zakwani I, Al-Hinai AT, Arafah M, Al Tamimi O, Shehab A, et al. Therapeutic lipid target achievements among highest risk patients: results from the CEPHEUS study in the Arabian gulf states. J Clin Lipidol. 2014;8(3):324-325.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.