| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 000, Number 000, March 2025, pages 000-000
Difference Analysis of N-Terminal B-Type Natriuretic Peptide, High-Sensitivity Troponin I, and Endothelin-1 Levels in Patients With Normotensive and Hypertensive Acute Heart Failure
Yose Ramda Ilhamia, e , Eryati Darwinb, Eva Decrolic, Efrida Efridad
aDepartment of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Andalas, General Hospital of Dr. M. Djamil, Padang 25162, Indonesia
bDepartment of Histology, Faculty of Medicine, Universitas Andalas, Padang, Indonesia
cDepartment of Internal Medicine, Faculty of Medicine, Universitas Andalas, General Hospital of Dr. M. Djamil, Padang, Indonesia
dDepartment of Clinical Pathology, Faculty of Medicine, Universitas Andalas, General Hospital of Dr. M. Djamil, Padang, Indonesia
eCorresponding Author: Yose Ramda Ilhami, Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Andalas, General Hospital of Dr. M. Djamil, Padang 25162, Indonesia
Manuscript submitted October 17, 2024, accepted March 13, 2025, published online March 18, 2025
Short title: Cardiac Biomarkers Level in AHF Patients
doi: https://doi.org/10.14740/cr1742
| Abstract | ▴Top |
Background: Acute heart failure (AHF) is a condition commonly affecting elderly patients. Heart failure is classified based on systolic blood pressure (SBP) into hypertensive (SBP ≥ 140 mm Hg), and normotensive (SBP < 140 mm Hg) categories. Differences in the pathophysiological mechanisms associated with each type of AHF may result in varying levels of biomarkers released by the heart during the episode, including N-terminal B-type natriuretic peptide (NT-proBNP), high-sensitivity (hs)-troponin I, and endothelin-1. Currently, there are no studies comparing the levels of cardiac biomarkers between normotensive and hypertensive AHF. Therefore, this study aimed to compare the levels of NT-proBNP, hs-troponin I, and endothelin-1 in patients with hypertensive and normotensive AHF.
Methods: A cross-sectional study was conducted in 104 patients with AHF (40 hypertensive, 64 normotensive) at M. Djamil General Hospital from August 2021 to November 2022. Clinical characteristics, hemodynamic parameters, and cardiac biomarker levels were assessed and compared between groups.
Results: Patients with hypertensive AHF had significantly higher sodium and chloride levels with lower urea levels. Echocardiographic assessment showed higher left ventricular ejection fraction (LVEF) (35.72% vs. 35.25%, P = 0.857), cardiac output (3.0 vs. 2.9 L/min, P = 0.669), and systemic vascular resistance (SVR) (2,276 vs. 2,200, P = 0.693), with lower tricuspid annular plane systolic excursion (TAPSE) (1.7 vs. 1.8 cm, P = 0.717), and estimated right atrial pressure (eRAP) > 8 (87.5% vs. 92.6%, P = 0.517) in normotensive AHF patients compared to hypertensive group, although there was no statistically significant difference between the two groups. The biomarkers test showed higher hs-troponin I levels (281 vs. 72.8 ng/L, P = 0.039) in normotensive AHF than those in hypertensive group. No significant differences were observed in endothelin-1 (12.12 vs. 12.02 pg/L, P = 0.510) and NT-proBNP levels (5,410 vs. 4,712 pg/mL, P = 0.122) between groups.
Conclusions: In patients with normotensive AHF, higher levels of hs-troponin I were observed, with no significant differences in other cardiac biomarkers. A higher proportion of males and a lower prevalence of hypertension as a risk factor were also noted in normotensive AHF, although these differences were not statistically significant.
Keywords: AHF; Normotensive; Hypertensive; NT-proBNP; High-sensitivity troponin I; Endothelin-1; Cardiac biomarker level
| Introduction | ▴Top |
Acute heart failure (AHF) is a condition with a high morbidity and mortality rate, particularly among elderly patients [1]. AHF can be categorized based on systolic blood pressure (SBP) into two groups: hypertensive (SBP ≥ 140 mm Hg) and normotensive (SBP < 140 mm Hg) [2]. The pathophysiological mechanisms differ between these two conditions. Normotensive AHF occurs due to fluid accumulation caused by cardiac dysfunction, which activates neurohormonal pathways [3]. In hypertensive AHF, with hypertrophic structural remodeling of the left ventricle (LV), there is an uncoupling of the ventricular-vascular relationship, so that LV has insufficient reserve to compensate for increased afterload and reduced venous capacitance results in fluid redistribution into the pulmonary circulation [4]. The diagnosis of AHF is based on the signs and symptoms of a rapid onset or worsening of heart failure (HF), which are confirmed through diagnostic examinations such as chest X-ray, lung ultrasound, and echocardiography. Several cardiovascular biomarkers, such as N-terminal B-type natriuretic peptide (NT-proBNP), endothelin-1, and high-sensitivity (hs)-troponin, can be used in AHF [5].
NT-proBNP is primarily generated by the ventricular wall of the heart and released when ventricular stretching happens. HF patients and other cardiac dysfunctions, such as myocardial infarction, can lead to an increase in this biomarker level. Higher level of NT-proBNP indicates more severe condition of AHF. Endothelin-1 is a peptide primarily produced by endothelial cells of blood vessels and cardiomyocytes. This peptide has prothrombotic and proinflammatory effects that play a role in the development of various cardiovascular diseases, including HF [6-8]. Increased endothelin-1 level has been known as the central pathogenesis of hypertension. Elevated levels of endothelin-1 in patients with AHF or chronic heart failure (CHF) are indicative of a higher risk of rehospitalization and mortality [9].
Troponin, a protein released by the heart in response to cardiac muscle damage, is also one of the cardiovascular biomarkers in HF [10]. While troponin can also be generated by skeletal muscle, there are isotopic distinctions between these two groups. Level of hs-troponin serves as a marker of cardiac muscle injury in HF and acute coronary syndrome (ACS), which is one of the most common causes of AHF. Increased levels of hs-troponin can be utilized for prognostic assessment, determining outcomes, and predicting mortality in patients with AHF and CHF [11, 12]. Therefore, hs-troponin can be a valuable biomarker in cases of AHF. The study comparing these three biomarkers in patients with normotensive and hypertensive AHF was not well established yet. This study aimed to evaluate hs-troponin I, endothelin-1, and NT-proBNP levels as potential cardiac biomarkers in patients with normotensive and hypertensive AHF.
| Materials and Methods | ▴Top |
Study population and design
This research was a cross-sectional analytical study conducted on AHF patients at Dr. M. Djamil Hospital from August 2021 to November 2022. Sampling was carried out using total sampling, considering the inclusion and exclusion criteria. The exclusion criteria in this study were AHF patients with complications from other diseases, including arrhythmias, patients with end-stage chronic kidney disease (CKD), and patients with sepsis. From a total of 139 patients treated with a diagnosis of AHF at the Integrated Heart Disease Installation of Dr. M. Djamil Hospital, 35 patients were excluded. Echocardiography was performed on a total of 104 patients in this study, all of whom had previously undergone initial treatment in the emergency room. These patients were then admitted to the Cardiovascular Care Unit (CVCU) for echocardiography and further treatment. This study was approved by the Research Ethical Committee of Medical Faculty Universitas Andalas, Padang, Indonesia (ethical clearance No. 423/UN.16.2/KEP-FK/2021). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Biomarker analysis
AHF patients who met the inclusion and exclusion criteria were evaluated for HF classification based on their medical history and clinical parameters. Venous blood samples were collected for hs-troponin I, NT-proBNP, and endothelin-1 analysis in plasma tubes containing heparin. The blood samples were centrifuged for 10 min, and aliquots were preserved in microcentrifuge tubes at -80 °C until required. The hs-troponin I levels were measured using the enzyme-linked immunosorbent assay (ELISA) method (utilizing the VIDAS high-sensitive troponin I kit from Biomerieux) with a detection limit of 1.3 - 3.2 ng/L, a coefficient of variation of 7.0% and a 99th percentile value of 19 ng/L. Endothelin-1 levels were assessed using the ELISA method (using the Human ET-1 ELISA kit from Elabscience), with a detection limit of 1.25 - 80 pg/mL and sensitivity of 0.75 pg/mL. Measurement of NT-proBNP concentration was performed using the ELISA method (VIDAS NT-proBNP), with a detection limit of 5 pg/mL, a cut-off value for acute onset of 300 pg/mL, and a coefficient of variation of ≤ 3.5%.
Statistical analysis
Data analysis was conducted using Statistical Package for the Social Sciences (SPSS) 26.0.0.1 software. Univariate analysis was done to describe basic characteristics, echocardiographic images, and levels of NT-proBNP, hs-troponin I and endothelin-1. Bivariate analysis was also performed with independent t-test to evaluate the relationship between independent and dependent variables if the data were normally distributed, and Mann Whitney if the data were not normally distributed. The independent variable was the type of AHF, while the dependent variables were basic characteristic, echocardiographic variables and levels of NT-proBNP, hs-troponin I and endothelin-1.
Results
The flowchart of the study was shown in Figure 1. From a total of 139 patients with AHF who met the inclusion criteria, 35 patients were excluded (15 because of arrhythmia, 11 because glomerular filtration rate (GFR) < 15 mL/min and nine patients because the cause was sepsis). From a total of 104 samples analyzed based on SBP, 64 patients with normotensive AHF and 40 patients with hypertensive AHF were obtained. An overview of the basic characteristics can be seen in Table 1. Based on the clinical characteristics of patients, it was found that in both groups, the elderly, males, and overweight were more common. The results in both groups showed that hypertension was the most common comorbidity, followed by ACS and diabetes mellitus. There were differences in therapy between the two groups; in the normotensive group, more diuretic therapy was administered, while in the hypertensive group, more angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) therapy was given but with no significant difference between two groups. The results of laboratory examinations also showed no significant differences between the two groups, except for higher sodium and chloride levels and lower urea levels found in hypertensive AHF patients.
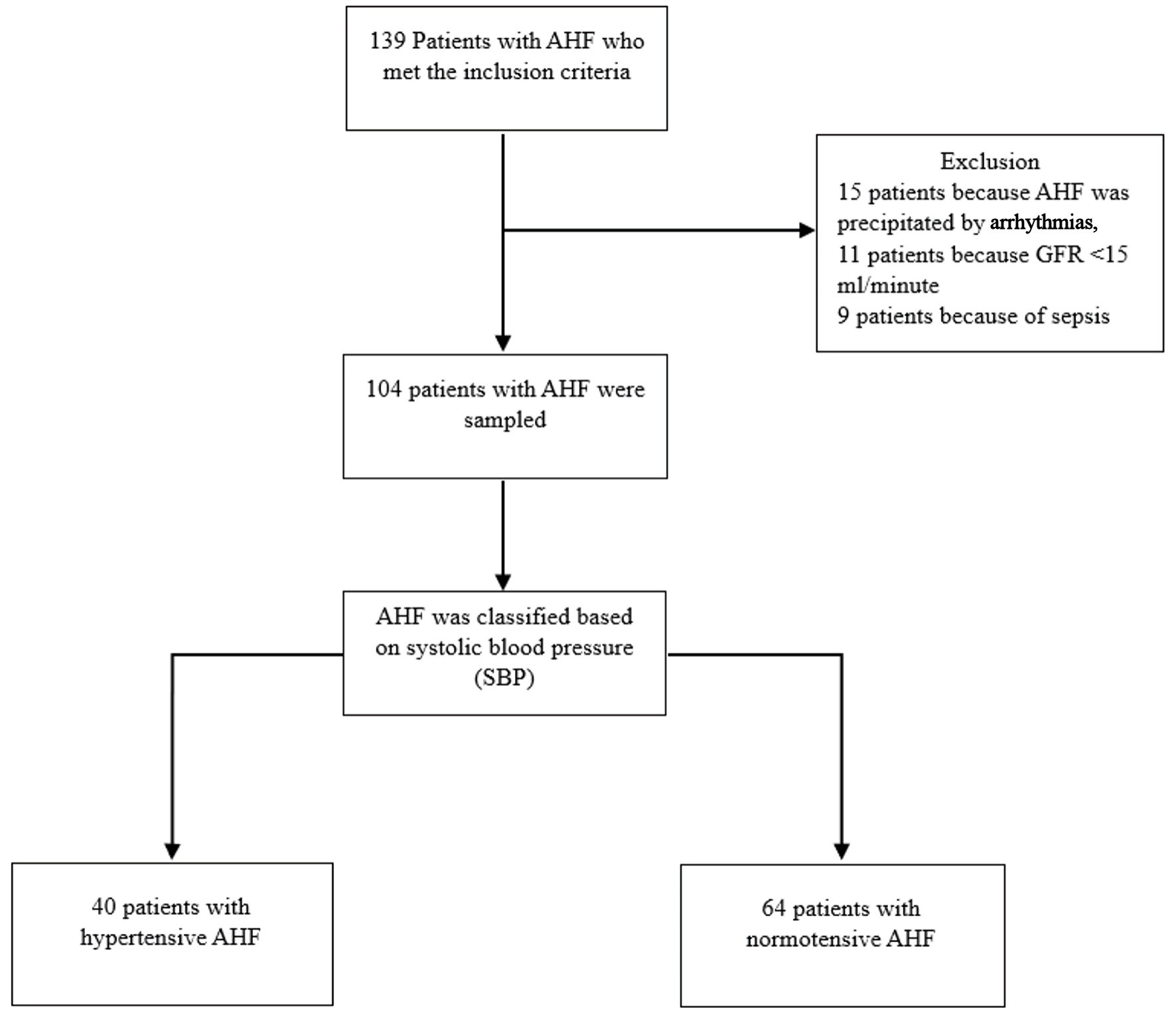 Click for large image | Figure 1. Flowchart of study. AHF: acute heart failure; SBP: systolic blood pressure; GFR: glomerular filtration rate. |
 Click to view | Table 1. Data Characteristics |
Hemodynamic features based on physical examination and echocardiography examination are shown in Table 2. In the hypertensive AHF group, there were significant differences, specifically higher systolic and diastolic pressures compared to the AHF normotensive group. The results of echocardiographic assessment showed that patients with normotensive AHF have higher value in left ventricular ejection fraction (LVEF), cardiac output, and systemic vascular resistance (SVR), but lower tricuspid annular plane systolic excursion (TAPSE) than hypertensive AHF group. There were no differences in estimated right atrial pressure (eRAP) findings in both groups.
 Click to view | Table 2. Hemodynamic Feature |
The description of NT-proBNP, hs-troponin I and endothelin-1 levels in the study is shown in Table 3. The results of the examination of cardiac biomarkers showed that hs-troponin I levels were significantly higher in the normotensive AHF group compared to the hypertensive AHF group. Endothelin-1 and NT-proBNP levels were also found higher in normotensive AHF group but with no significant difference.
 Click to view | Table 3. Overview of High-Sensitivity Troponin I and Endothelin-1 Levels |
| Discussion | ▴Top |
In this study, we compared the characteristics, hemodynamic profiles, and cardiac biomarker levels between two groups of HF classified by SBP, namely normotensive and hypertensive AHF. It was found that the normotensive AHF group exhibited more than the hypertensive AHF group. Similar findings were reported in a study, which involved 1,152 patients diagnosed with AHF. The study revealed that 667 patients (58%) belonged to the normotensive AHF group [13]. The pathophysiology of normotensive AHF is primarily caused by cardiac dysfunction [3]. Cardiac dysfunction triggers various processes such as neurohormonal activation. Neurohormonal activation, involving the renin-angiotensin-aldosterone system (RAAS), sympathetic nervous system, and arginine-vasopressin system, aims to counteract the negative hemodynamic effects in HF, such as decreased tissue perfusion. This neurohormonal activation results in increased sodium retention in the blood, leading to fluid retention and ultimately congestion, a key clinical symptom of AHF [14]. Untreated neurohormonal activation can progress to myocardial dysfunction and other complications, including myocardial hypertrophy, myocardial apoptosis, reduced myocardial contractility, and decreased myocardial responsiveness to beta-adrenergic stimuli [15]. Myocardial damage contributes to a decrease in ejection fraction and cardiac output, leading to decreased blood pressure. Regardless of the underlying cause of cardiac dysfunction, this process is central to AHF [15]. This explains why normotensive AHF is more prevalent than hypertensive AHF.
Most patients with hypertensive AHF have a history of chronic hypertension and HF [16]. Chronic hypertension, persisting over a long time, can cause damage in various organs, one of which is the kidney (an organ that is very instrumental in the process of regulating blood pressure through the RAAS). This system regulates blood pressure by controlling blood circulation volume, resistance, and vascular tone. Additionally, the process of regulating blood pressure by RAAS also involves regulating ion excretion, especially sodium ions. Damage to the kidneys due to chronic hypertension causes disturbances in this system, resulting in disruptions in blood pressure regulation, including a decrease in sodium ion excretion [17]. In addition, decreased chloride levels are observed in HF patients with low cardiac index. Patients with HF and low cardiac index experience increased neurohormonal activation, which will lead to hypoperfusion, systemic vasoconstriction and finally fluid accumulation and hemodilution. Hemodilution will lead to decreased chloride levels. Higher use of diuretics in normotensive AHF patients may also contribute to differences in electrolyte levels. These conditions lead to lower sodium and chloride ion levels in patients with normotensive compared to hypertensive AHF patients [18]. Blood gas analysis revealed a normal pH with decreased pCO2 and bicarbonate ion levels in both groups. In AHF conditions, peripheral hypoperfusion typically occurs, which can lead to metabolic acidosis or a mixed type. The reduction in bicarbonate ions during metabolic acidosis induces the body to release more CO2 through hyperventilation, resulting in hypocapnia [18].
Echocardiographic results showed a decreased LVEF (heart failure with reduced ejection fraction (HFrEF)) in both groups; however, no significant difference was noted between them. This finding contrasts with previous studies, which indicated that patients with hypertensive AHF had normal LVEF (heart failure with preserved ejection fraction, HFpEF), while the normotensive AHF group demonstrated HFrEF [19]. LV systolic dysfunction, characterized by reduced LVEF, leads to diminished cardiac output and blood pressure, resulting in a normotensive presentation. This also suggests a more severe degree of cardiomyocyte damage and a later stage of HF in the normotensive AHF group compared to hypertensive AHF [20]. Cardiac output values were found to be decreased in both groups, with the normotensive AHF group exhibiting slightly higher values than the hypertensive AHF group. As previously mentioned, normotensive AHF patients generally experience a reduction in LVEF, which contributes to decreased cardiac output. In contrast, hypertensive AHF patients typically maintain normal LVEF [13]. However, the observed decrease in cardiac output in this study may be attributed to increased afterload associated with hypertensive AHF conditions. SVR measurements were elevated in both groups, with the normotensive AHF group showing higher SVR. The increase in SVR in normotensive AHF serves as a compensatory mechanism to counteract decreased cardiac output and maintain SBP [21]. On the other hand, increased SVR in hypertensive AHF arises from vasoconstriction and ventricular-vascular mismatch, which are the primary pathophysiological mechanisms underlying hypertensive AHF. Additionally, TAPSE values were lower in the normotensive AHF group compared to the hypertensive AHF group. As previously discussed, the more severe damage and advanced stage of HF in normotensive AHF contribute to a greater reduction in right ventricular (RV) contractility [20].
Cardiac troponins, especially hs-troponin, are biomarkers released by the heart when myocardial cell damage or death occurs [22]. These biomarkers are commonly used as one of the criteria in the diagnosis of acute myocardial infarction (AMI) [23]. Measurement of hs-troponin levels can also be used in AHF patients to exclude myocardial ischemia as a precipitating factor for AHF. However, AHF patients may also have elevated cardiac troponin levels without the presence of myocardial ischemia [1]. A possible mechanism is the imbalance between the need and supply of oxygen to the heart and damage to the myocardium due to neurohormonal responses, oxidative stress, and inflammation [24]. Increased hs-troponin levels in patients with AHF are associated with increased parietal strain in myocardium and increased cardiac filling pressure. Prognostically, increased hs-troponin levels were independent predictors of hospitalization for HF and cardiovascular death, so optimizing AHF therapy using hs-troponin levels will decrease the pathogenic mechanisms associated with AHF [18]. Patients with normotensive AHF have higher cardiac hemodynamic stress and myocardial damage compared to hypertensive AHF, which is evidenced by the higher prevalence of HFrEF in patients with normotensive AHF [20]. More damage in normotensive AHF leads to higher hs-troponin levels in normotensive AHF compared to hypertensive AHF [25]. However, high levels of hs-troponin in this study cannot exclude the possibility of myocardial ischemia as a precipitating factor for AHF.
Endothelin-1 is a biomarker that is not only released by myocardial cells but also endothelial cells. Endothelin-1 causes cardiac hypertrophy and has pro-inflammatory and pro-fibrotic effects in HF patients. Endothelin-1 is known as a predictor and prognostic marker in patients with either AHF or CHF [6-8]. Endothelin-1 is commonly used as a prognostic factor in HF patients and is associated with 180-day mortality in patients with acute decompensated heart failure (ADHF). In patients with symptomatic HF, elevated endothelin-1 levels were predictive of mortality [26, 27]. In our research, there was no significant difference in the normotensive AHF group compared with hypertensive AHF group, indicating the same level of prognosis in both groups.
NT-proBNP is a biomarker produced by the ventricular wall muscle, particularly in the LV. This biomarker is primarily generated in response to mechanical stress on the ventricular wall caused by pressure and volume overload [28]. Additionally, NT-proBNP is produced under conditions of myocardial ischemia and neurohormonal activation [29]. The examination of NT-proBNP should be conducted in all patients exhibiting symptoms of worsening HF or AHF as a form of diagnostic support [30]. According to the European Society of Cardiology (ESC) guidelines, a diagnosis of HF can be made if NT-proBNP levels are > 450 pg/mL in patients younger than 50 years, > 900 pg/mL in patients aged 50 to 75 years, and > 1,800 pg/mL in patients older than 75 years [31]. The increase in NT-proBNP serves to maintain homeostatic conditions through various mechanisms, including arterial vasodilation, natriuresis, diuresis, and counteracting the effects of the activation of RAAS, sympathetic nervous system, and endothelin. NT-proBNP also exhibits antihypertrophic and antifibrotic effects [32]. Several studies have demonstrated that there is a consistent increase in BNP and NT-proBNP levels in patients with AHF, and the elevation of these biomarkers is closely associated with the severity of the disease [16]. This indicates that patients with a higher New York Heart Association (NYHA) classification tend to have higher NT-proBNP levels, reflecting a more severe degree of HF.
Based on the findings of this study, normotensive AHF patients have more severe clinical conditions, as supported by laboratory and echocardiographic assessments showing more pronounced abnormalities. In this regard, normotensive AHF patients must be managed more comprehensively. Besides having to carry out treatment for AHF, it is also necessary to monitor for complications that may occur, including hypotension due to administration of drugs for AHF. These patients who have a history of other diseases such as diabetes mellitus and CKD must also continue receiving treatment for these comorbidities in addition to treatment for their AHF.
Limitations
This study was a single-center observational study which has several confounding factors, including no adjustment for risk factors and no subgroup analysis related to previous history of HF in patients. In our study, the hypotensive AHF group was included with the normotensive AHF group, so some results regarding hypotensive AHF cannot be concluded. Another limitation was the slight differences in timing between blood pressure measurement at admission and echocardiographic assessments, which can produce bias in the result of this study. Larger, randomized trials with follow-up periods to validate the findings are recommended for further studies.
Conclusions
In patients with normotensive AHF, there were decreased levels of sodium and chloride ion, and higher levels of urea and hs-troponin I. A higher proportion of males and a lower prevalence of hypertension as a risk factor were also observed in the normotensive AHF group, although these differences were not statistically significant.
AHF: acute heart failure; NT-proBNP: N-terminal B-type natriuretic peptide.
Acknowledgments
The authors thank everyone who contributed to and assisted with the data collection in this study.
Financial Disclosure
This research was partially funded by the Faculty of Medicine at Universitas Andalas and supplemented with personal funds.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
All subjects provided written informed consent.
Author Contributions
YRI conducted the design, sample collection and analysis, manuscript preparation and critical editing. E. Darwin, E. Decroli, and EE carefully supervised this manuscript preparation and writing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ACEI: angiotensin-converting enzyme inhibitor; ACS: acute coronary syndrome; ADHF: acute decompensated heart failure; AHF: acute heart failure; AMI: acute myocardial infarction; ARB: angiotensin receptor blocker; BMI: body mass index; CCT: creatinine clearance test; CHF: chronic heart failure; CKD: chronic kidney disease; ELISA: enzyme linked immunosorbent assay; eRAP: estimated right atrial pressure; ESC: European Society of Cardiology; HF: heart failure; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; hs-troponin: high-sensitivity troponin; LV: left ventricle; LVEF: left ventricular ejection fraction; MRA: mineralocorticoid receptor antagonist; NT-proBNP: N-terminal B-type natriuretic peptide; NYHA: New York Heart Association; RAAS: renin-angiotensin-aldosterone system; RBG: random blood glucose; RV: right ventricle; SBP: systolic blood pressure; SPSS: Statistical Package for the Social Sciences; SVR: systemic vascular resistance; TAPSE: tricuspid annular plane systolic excursion
| References | ▴Top |
- Arrigo M, Parissis JT, Akiyama E, Mebazaa A. Understanding acute heart failure: Pathophysiology and diagnosis. Eur Hear Journal. 2016;18(Supplement G):G11-G18.
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726.
doi pubmed - Arrigo M, Rudiger A. Acute heart failure: from pathophysiology to optimal treatment. Cardiovasc Med. 2017;20(10):229-234.
- Viau DM, Sala-Mercado JA, Spranger MD, O'Leary DS, Levy PD. The pathophysiology of hypertensive acute heart failure. Heart. 2015;101(23):1861-1867.
doi pubmed - Piek A, Du W, de Boer RA, Sillje HHW. Novel heart failure biomarkers: why do we fail to exploit their potential? Crit Rev Clin Lab Sci. 2018;55(4):246-263.
doi pubmed - Jankowich M, Choudhary G. Endothelin-1 levels and cardiovascular events. Trends Cardiovasc Med. 2020;30(1):1-8.
doi pubmed - Matsubara TJ, Fujiu K. Endothelin-1 and atrial cardiomyopathy. Int Heart J. 2019;60(2):238-240.
doi pubmed - Omland T. Targeting the endothelin system: a step towards a precision medicine approach in heart failure with preserved ejection fraction? Eur Heart J. 2019;40(45):3718-3720.
doi pubmed - Yeoh SE, Docherty KF, Campbell RT, Jhund PS, Hammarstedt A, Heerspink HJL, Jarolim P, et al. Endothelin-1, outcomes in patients with heart failure and reduced ejection fraction, and effects of dapagliflozin: findings from DAPA-HF. Circulation. 2023;147(22):1670-1683.
doi pubmed - Nadar SK, Shaikh MM. Biomarkers in routine heart failure clinical care. Card Fail Rev. 2019;5(1):50-56.
doi pubmed - Spinar J, Jarkovsky J, Spinarova L, Mebazaa A, Gayat E, Vitovec J, Linhart A, et al. AHEAD score—Long-term risk classification in acute heart failure. Int J Cardiol. 2016;202:21-26.
doi pubmed - Roberts E, Ludman AJ, Dworzynski K, Al-Mohammad A, Cowie MR, McMurray JJ, Mant J, et al. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ. 2015;350:h910.
doi pubmed - Kozhuharov N, Michou E, Wussler D, Belkin M, Heinisch C, Lassus J, Siirila-Waris K, et al. Quantifying hemodynamic cardiac stress and cardiomyocyte injury in normotensive and hypertensive acute heart failure. Biomedicines. 2024;12(5):1099.
doi pubmed - Nijst P, Verbrugge FH, Grieten L, Dupont M, Steels P, Tang WHW, Mullens W. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol. 2015;65(4):378-388.
doi pubmed - Ponikowski P, Jankowska EA. Pathogenesis and clinical presentation of acute heart failure. Rev Esp Cardiol (Engl Ed). 2015;68(4):331-337.
doi pubmed - Lasica R, Djukanovic L, Vukmirovic J, Zdravkovic M, Ristic A, Asanin M, Simic D. Clinical review of hypertensive acute heart failure. Medicina (Kaunas). 2024;60(1):133.
doi pubmed - Emmens JE, Ter Maaten JM, Matsue Y, Figarska SM, Sama IE, Cotter G, Cleland JGF, et al. Worsening renal function in acute heart failure in the context of diuretic response. Eur J Heart Fail. 2022;24(2):365-374.
doi pubmed - Gherasim L. Troponins in heart failure - a perpetual challenge. Maedica (Bucur). 2019;14(4):371-377.
doi pubmed - Garus M, Zdanowicz A, Fudim M, Zymlinski R, Niewinski P, Paleczny B, Rosiek-Biegus M, et al. Clinical determinants and prognostic significance of hypocapnia in acute heart failure. Sci Rep. 2022;12(1):16889.
doi pubmed - Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, Stough WG, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296(18):2217-2226.
doi pubmed - Murphy SP, Ibrahim NE, Januzzi JL, Jr. Heart failure with reduced ejection fraction: a review. JAMA. 2020;324(5):488-504.
doi pubmed - Cotter G, Moshkovitz Y, Kaluski E, Milo O, Nobikov Y, Schneeweiss A, Krakover R, et al. The role of cardiac power and systemic vascular resistance in the pathophysiology and diagnosis of patients with acute congestive heart failure. Eur J Heart Fail. 2003;5(4):443-451.
doi pubmed - Sabbah HN. Pathophysiology of acute heart failure syndrome: a knowledge gap. Heart Fail Rev. 2017;22(6):621-639.
doi pubmed - Adler Y, Charron P, Imazio M, Badano L, Baron-Esquivias G, Bogaert J, Brucato A, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921-2964.
doi pubmed - Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, et al. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation. 2017;135(22):e1054-e1091.
doi pubmed - Grodin JL, Neale S, Wu Y, Hazen SL, Tang WH. Prognostic comparison of different sensitivity cardiac troponin assays in stable heart failure. Am J Med. 2015;128(3):276-282.
doi pubmed - He X, Guo H, Xu J. Endothelin 1: a potential prognostic biomarker for heart failure with preserved ejection fraction and pulmonary hypertension? Cardiology. 2020;145(4):262.
doi pubmed - Gottlieb SS, Harris K, Todd J, Estis J, Christenson RH, Torres V, Whittaker K, et al. Prognostic significance of active and modified forms of endothelin 1 in patients with heart failure with reduced ejection fraction. Clin Biochem. 2015;48(4-5):292-296.
doi pubmed - Goetze JP, Bruneau BG, Ramos HR, Ogawa T, de Bold MK, de Bold AJ. Cardiac natriuretic peptides. Nat Rev Cardiol. 2020;17(11):698-717.
doi pubmed - Volpe M, Rubattu S, Burnett J, Jr. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. 2014;35(7):419-425.
doi pubmed - Tsutsui H, Albert NM, Coats AJS, Anker SD, Bayes-Genis A, Butler J, Chioncel O, et al. Natriuretic peptides: role in the diagnosis and management of heart failure: a scientific statement from the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. Eur J Heart Fail. 2023;25(5):616-631.
doi pubmed - Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21(6):715-731.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.