| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 000, Number 000, June 2025, pages 000-000
Clinical Significance of Serum Total Testosterone Concentration in Japanese Elderly Women With Pre-Heart Failure With Preserved Ejection Fraction
Takashi Hitsumoto
Hitsumoto Medical Clinic, Shimonoseki City, Yamaguchi 750-0025, Japan
Manuscript submitted May 2, 2025, accepted May 28, 2025, published online June 9, 2025
Short title: Testosterone and Pre-HFpEF in Elderly Women
doi: https://doi.org/10.14740/cr2084
| Abstract | ▴Top |
Background: Currently, limited information is available regarding testosterone in women with heart failure with preserved ejection fraction (HFpEF). This cross-sectional study aimed to elucidate the clinical significance of serum total testosterone concentration (T-T) in Japanese elderly women with pre-HFpEF, a condition that develops before the onset of HF.
Methods: A total of 232 elderly women outpatients with pre-HFpEF (mean age ± standard deviation, 75 ± 7 years) were enrolled. Relationships between T-T and other clinical parameters were investigated. The definition of pre-HFpEF in this study was that patients with left ventricular ejection fraction of 50% or more and E/e' ratio as a marker of left ventricular function greater than 9 on echocardiography had no HF symptoms and no history of HF hospitalization.
Results: There was a significantly negative correlation between T-T and the E/e' ratio (r = -0.24, P < 0.001), and low T-T was significantly related to coronary artery disease. A significant correlation was observed between T-T and biomarkers such as blood brain natriuretic peptide concentration (r = -0.31, P < 0.001), serum high-sensitivity cardiac troponin T concentration (r = -0.33, P < 0.001), and the derivatives-reactive oxygen metabolites test, an oxidative stress marker (r = -0.46, P < 0.001). Furthermore, multiple regression analysis identified that the factors described above were selected as independent variables for T-T as a dependent variable.
Conclusions: This cross-sectional study indicated that low T-T levels reflect unfavorable pathophysiological conditions in Japanese elderly women with pre-HFpEF. To clarify the relevance of T-T as a predictive indicator for the onset of cardiovascular diseases, including HF incidence in elderly women with pre-HFpEF, future prospective studies, including interventional treatments, should be conducted.
Keywords: Testosterone; Pre-heart failure with preserved ejection fraction; Left ventricular diastolic dysfunction; Brain natriuretic peptide; High-sensitivity cardiac troponin T; Coronary artery disease; Oxidative stress
| Introduction | ▴Top |
In recent years, the prevalence of heart failure (HF) has been steadily increasing not only among Westerners but also among Asians, driven by the increased life expectancy and other factors. Consequently, the growing number of older patients with HF in clinical settings has led to the condition being described as a “heart failure pandemic” [1, 2]. Furthermore, the prognosis of heart failure with preserved ejection fraction (HFpEF) was found to be as poor as that for HF with reduced ejection fraction in several studies [3, 4]. According to recent clinical research, sodium glucose cotransporter 2 (SGLT2) inhibitor intervention improves HFpEF [5-7]. However, residual risks remain even with SGLT2 inhibitor, and therefore, additional treatment strategies need to be considered to enhance the prognosis of patients with HFpEF. Additionally, it has been observed that the prognosis is poor following stage C of the HF stage classification, when HF symptoms begin to develop [8, 9]. Therefore, early intervention is considered necessary for managing HF at an earlier stage. From this perspective, it is clinically important to explore novel treatment strategies for HFpEF during stages in which patients do not yet exhibit subjective symptoms of HF.
Although few studies have examined the relationship between testosterone levels and HFpEF, several reports indicate a significant relationship between HFpEF development or prognosis and low blood testosterone concentration in men [10, 11]. However, only one study has examined the association between serum total testosterone concentration (T-T) and the incidence of HFpEF in women in the United States, and it found no statistically significant relationship [11]. In addition, no studies to date have examined the relationship between T-T and HFpEF in Asian women. In this cross-sectional study, the author examined the clinical significance of T-T in Japanese elderly women with pre-HFpEF, which occurs prior to the onset of HF.
| Materials and Methods | ▴Top |
Patients
Among the outpatients of elderly women aged 65 years and older who visited the Hitsumoto Medical Clinic from March 2023 to February 2025, 232 consecutive pre-HFpEF patients with all clinical parameters, including T-T, were enrolled. T-T was evaluated employing a commercial kit (ARCHITECT Estradiol II, Chicago, IL, USA), and the correlation between T-T and other clinical parameters was analyzed. In this study, pre-HFpEF was defined as a left ventricular ejection fraction of 50% or more, an E/e' ratio ≥ 9 on echocardiography as a marker of diastolic dysfunction, absence of HF symptoms, and no history of HF-related hospitalization. The cutoff value of 9 for the E/e' ratio in this study was determined by consulting previous studies; this cutoff value and the score using this cutoff value were found to be related to the prognosis of HF [12-14].
Ethical considerations
This study was conducted in accordance with the Declaration of Helsinki and the ethical standards of institutions responsible for human research. Informed consent was obtained from all patients participating in this study, and the study protocol was approved by the Institutional Review Board of Hitsumoto Medical Clinic (date of approval: February 17, 2023; approval number: HMC-2023-2R-1).
The evaluation of clinical parameters
Various clinical parameters were evaluated, including conventional cardiovascular risk factors, presence of coronary artery disease (CAD) and atrial fibrillation, echocardiography outcomes, kidney function, blood concentrations of brain natriuretic peptide (BNP) and serum high-sensitivity cardiac troponin T (hs-cTnT), as well as markers of inflammation and oxidative stress. Classic risk factors for cardiovascular disease were defined in accordance with previous reports [15]. In this study, CAD was defined as the presence of angina pectoris and/or myocardial infarction managed either with coronary revascularization or oral medication alone. Paroxysmal and chronic atrial fibrillation were considered as types of atrial fibrillation. Echocardiography was performed utilizing standard procedures, and left ventricular wall thickness, left ventricular extended period diameter, left ventricular ejection fraction, left atrial dimension, and E/e' ratio were measured. Fasting blood samples were collected from peripheral veins following a routine protocol, and blood glucose-related indicators, lipid-related indices, kidney function indicators, BNP, hs-cTnT, as well as inflammatory and oxidative stress indices were evaluated. The estimated glomerular filtration rate (eGFR), a marker of kidney function, was calculated using Japanese criteria [16]. A commercial kit was used for assessing BNP (SHIONOSPOT Reader; Shionogi & Co., Osaka, Japan) and hs-cTnT (Roche Diagnostics, Switzerland) levels. Serum high-sensitivity C-reactive protein (hs-CRP) concentration, a marker of inflammation, was measured using the latex nephelometry technique. The derivatives-reactive oxygen metabolites (d-ROMs) test, an oxidative stress marker, was ascertained using a commercial kit (Diacron, Grosseto, Italy) [17]. Use of oral medications, such as renin-angiotensin system (RAS) inhibitors (renin-angiotensin inhibitors or angiotensin receptor blockers), β-blockers, statins, and SGLT2 inhibitors, was also documented. However, no patients in the study were receiving mineralocorticoid receptor antagonists and diuretics.
Statistical analysis
The commercialized software Stat View-J version 5.0 (HULINKS Inc., Tokyo, Japan) and MedCalc for Windows (MedCalc Software, Ostend, Belgium) were employed for performing statistical analysis. Continuous variables are expressed in terms of mean and standard deviation or median (interquartile range). Simple regression analysis was performed using the Spearman rank correlation, and multivariate analysis was performed using multiple regression analysis. Multiple regression analysis was performed for T-T as a dependent variable. Eight explanatory variables, age, CAD, E/e' ratio, eGFR, BNP, hs-cTnT, hs-CRP, and d-ROMs test, were selected based on their significant correlations with T-T in univariate analysis. A P-value < 0.05 was considered statistically significant.
| Results | ▴Top |
Patient characteristics
The patient characteristics are presented in Table 1. The mean age of the participants was 75 years. The lowest T-T value was 7.7 ng/dL, the highest was 69.0 ng/dL, and the median was 26.2 ng/dL. There were 53 (23%) CAD patients. The median value of the E/e' ratio was 14.5. Among those receiving oral medications, SGLT2 inhibitor use was noted in 16 patients (7%).
 Click to view | Table 1. Baseline Characteristics of the Study Population |
Correlation between T-T and E/e'
Figure 1 depicts the correlation between T-T and E/e' ratio. A significant negative correlation was observed between T-T and E/e' ratio (r = -0.24, P < 0.001).
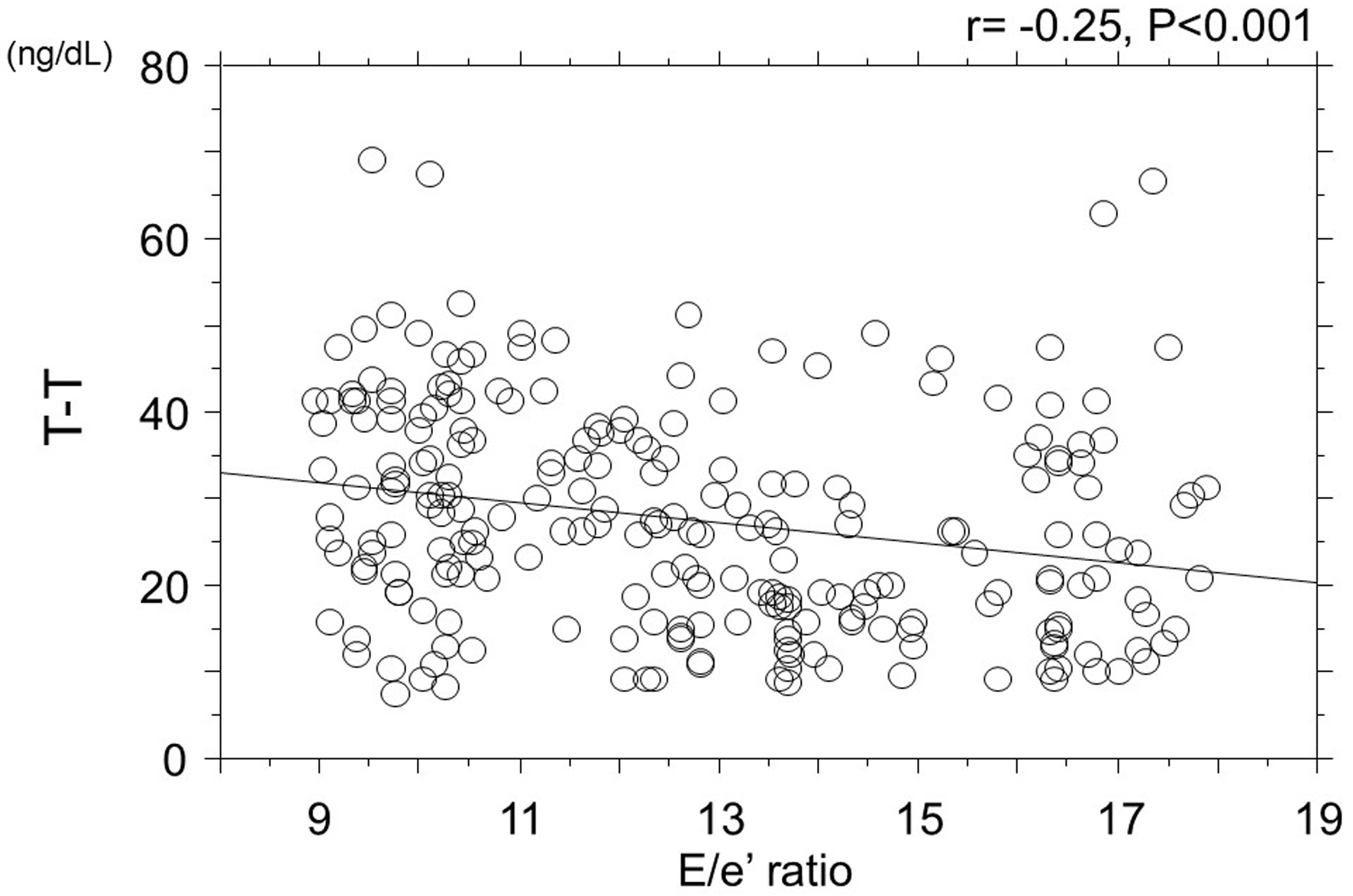 Click for large image | Figure 1. Correlation between T-T and E/e' ratio. T-T: blood total testosterone concentration. |
Correlations between T-T and various clinical parameters
Table 2 illustrates the correlation between T-T and various clinical parameters. T-T exhibited significantly negative correlations with age, presence of CAD, BNP, hs-cTnT, hs-CRP, and d-ROMs tests. However, T-T demonstrated a significantly positive correlation with eGFR. There was no significant association between SGLT2 inhibitor use and T-T.
 Click to view | Table 2. Correlation Between T-T and Various Clinical Characteristics |
Multivariate analysis
The results of the multiple regression analysis are illustrated in Table 3. Among the eight explanatory factors, five (i.e., d-ROMs test, BNP, hs-cTnT, E/e' ratio, and CAD) were selected as independent variables, with T-T serving as the dependent variable.
 Click to view | Table 3. Multivariate Analysis for T-T |
| Discussion | ▴Top |
This study aimed to clarify the clinical significance of T-T in Japanese elderly women with pre-HFpEF. T-T exhibited a significantly negative correlation with the E/e' ratio, a marker of left ventricular diastolic function. Furthermore, an increase in the E/e' ratio was identified as an independent predictor of low T-T levels. Cardiac biomarkers such as BNP and hs-cTnT were also independently associated with low T-T, and an independent correlation was noted between CAD and low T-T. However, the d-ROMs test, an oxidative stress marker, was identified as the strongest predictor for low T-T.
Relationships between testosterone and left ventricular diastolic function
Several researchers have emphasized the relevance of left ventricular diastolic dysfunction in HFpEF pathogenesis [18-20]. In this study, the E/e' ratio, an index of diastolic function, was also employed as a diagnostic criterion for pre-HFpEF. In addition, several clinical reports have indicated that low blood testosterone concentrations are significantly associated with left ventricular diastolic dysfunction in men, as assessed by echocardiography [21-23]. However, although a relatively weak negative correlation between T-T and the E/e' ratio was detected in this study, it was statistically significant, and an independent relationship between the two markers was observed. Therefore, it is considered that low blood testosterone concentration is associated with left ventricular diastolic dysfunction even in elderly women with pre-HFpEF. To clarify the direct effects of testosterone on left ventricular diastolic function, it is desirable to screen elderly women with pre-HFpEF who have low T-T and investigate whether testosterone replacement therapy contributes to the improvement of the E/e' ratio or other left ventricular diastolic function marker and ultimately prevents the onset of HF in these patients.
Testosterone and blood cardiac biomarkers
BNP increases when there is left ventricular overload. In addition, several researchers have indicated that BNP can be a prognostic indicator for HFpEF, similar to HF-related rehospitalization [24-26]. Furthermore, previous studies have also reported that BNP can be employed as an index for predicting initial HF-related hospitalization [27, 28]. Therefore, the independent relationship between BNP and T-T in the study results indicates that T-T may be a predictor for the HF onset through increased left ventricular load in elderly women with pre-HFpEF. Multiple studies have indicated the importance of arterial dysfunction in the pathogenesis of HFpEF [29-31]. In addition, there are reports demonstrating a significant association between low T-T and arterial dysfunction in postmenopausal women [32-34]. In this study, the evaluation of arterial function was not conducted; however, arterial dysfunction associated with decreased testosterone levels may lead to an increase in BNP due to left ventricular overload caused by increased afterload. Future research focusing on the relationship between testosterone and arterial function is required.
Hs-cTnT is used clinically as a biomarker to ascertain the degree of myocardial injury. In addition, numerous studies have demonstrated the prognostic significance of hs-cTnT in patients with HFpEF [35, 36]. Basic research has identified several mechanisms by which testosterone induces myocardial damage [37, 38]. Chung et al revealed that testosterone inhibits myofibroblast differentiation induced by transforming growth factor-β1, as well as cardiac fibroblast migration and proliferation [37]. Tsang et al demonstrated that testosterone stimulates α(1)-adrenergic receptors to protect the heart of rats exposed to ischemia [38]. Thus, the results of these basic studies and this cross-sectional study can be interpreted as indicating that low testosterone levels affect the progression of myocardial damage in elderly women with pre-HFpEF. To confirm this hypothesis, further prospective study is required to confirm prognosis of elderly women with HFpEF using T-T and hs-cTnT.
Relationships between testosterone and CAD
Several clinical studies have reported that the presence of CAD is a poor prognostic factor for patients with HFpEF [39, 40]. However, the relationship between CAD and testosterone levels in women remains controversial. Das et al reported that high T-T levels were significantly associated with the development of CAD [41]. In contrast, Kaczmarek et al reported that decreased testosterone levels are significantly linked to CAD [42]. In this study, T-T was significantly lower in patients with CAD than in non-CAD patients. Furthermore, an independent association was observed between CAD and low T-T levels. Thus, the results of this study are consistent with the report of Kaczmarek et al and indicate that low T-T also adversely influences the prognosis of elderly women with pre-HFpEF from the perspective of coronary artery atherosclerosis. Researchers highlight the importance of inflammation brought on by low testosterone as a contributing factor to the finding that male patients with low testosterone levels are also at risk of developing CAD [43, 44]. Although this cross-sectional study did not directly evaluate inflammatory markers, the findings suggest that inflammation may contribute to the significant association between low T-T and CAD in the study population. In the future, it is hoped that the relationship between T-T and inflammation in elderly women with pre-HFpEF will be clarified, and further prospective examination will be conducted to ascertain the benefits of interventions such as testosterone replacement therapy and lifestyle modifications.
Testosterone and oxidative stress
Research has highlighted that oxidative stress is closely linked to HFpEF pathogenesis [9, 45, 46]. In addition, several reports have reported relationships between d-ROMs tests, which are oxidative stress markers, and HF [27, 47, 48]. Hirata et al examined the association between the d-ROMs test and hospitalized HFpEF patients and reported that patients with elevated d-ROMs test parameters experienced significantly more cardiovascular events and HF-related readmissions than those with lower parameters and a poor prognosis [47]. In addition, the independent relationship between low T-T and d-ROMs test in the results of this cross-sectional study indicates that elderly women with pre-HFpEF and low blood testosterone concentration are at an increased risk of developing cardiovascular disease, including the initial onset of HF, not just progression to HFpEF after hospitalization. Zhang et al reported that low testosterone contributed to increased oxidative stress in cardiomyocytes [49]. Therefore, testosterone replacement may be effective for elderly women with pre-HFpEF for lowering oxidative stress. However, in this cross-sectional study, the mechanism by which oxidative stress is involved in the decrease of T-T may also be considered. Therefore, it is desirable to investigate whether antioxidant therapy can promote an increase in T-T and subsequently aid in preventing cardiovascular disease, including the onset of HF.
Limitations
The limitations of this study are described below. First, this study did not evaluate other relevant sex hormones such as dehydroepiandrosterone sulfate, sex hormone binding globulin, and estradiol. According to previous research, dehydroepiandrosterone sulfate in particular plays a major role in the development of HFpEF in postmenopausal females [11]. In the multivariate analysis conducted in this study, including these hormone values as analysis factors may have eliminated the relationship between T-T and various HF-related indicators. In the future, it would be desirable to include the evaluation of related hormones, such as dehydroepiandrosterone sulfate, in further investigations. Second, this study used the E/e' ratio for clinical simplicity. However, there are multiple indicators for left ventricular diastolic function, such as HFA-PEFF scores, left atrial volume index, tricuspid regurgitation velocity, and global longitudinal strain. In the future, it is hoped that the relationship between these indicators and T-T will be explored to elucidate the association between left ventricular diastolic function and T-T. Third, in this study, a decrease in T-T was associated with various indicators related to HFpEF in elderly women with pre-HFpEF. However, since this study is a cross-sectional analysis, it is necessary to verify through future prospective studies how cases of low T-T progress over time, how they develop HF, and what their severity is. Furthermore, it is necessary to conduct risk stratification based on T-T using a large number of cases and investigate whether interventions such as testosterone replacement therapy and lifestyle improvements for elderly women with low T-T can improve the prognosis of pre-HFpEF.
Conclusions
This cross-sectional study indicated that low T-T levels reflect unfavorable pathophysiological conditions in Japanese elderly women with pre-HFpEF. To clarify the relevance of T-T as a predictive indicator for the onset of cardiovascular diseases, including HF incidence in elderly women with pre-HFpEF, future prospective studies, including interventional treatments, should be conducted.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from all patients who participated in this study.
Author Contributions
Takashi Hitsumoto contributed to the research planning, data acquisition and analysis, and manuscript writing and editing.
Data Availability
The author declares that data supporting the findings of this study are available within the article.
Abbreviations
BNP: brain natriuretic peptide; CAD: coronary artery disease; d-ROMs: derivatives-reactive oxygen metabolites; eGFR: estimated glomerular filtration rate; HFpEF: heart failure with preserved ejection fraction; hs-CRP: high-sensitivity C-reactive protein; hs-cTnT: high-sensitivity cardiac troponin T; RAS: renin-angiotensin system; SGLT2: sodium glucose cotransporter 2; T-T: total testosterone concentration
| References | ▴Top |
- Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63(12):1123-1133.
doi pubmed - Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015;17(9):884-892.
doi pubmed - Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, Yokota T, Goto D, Yokoshiki H, Kato N, et al. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J. 2009;73(10):1893-1900.
doi pubmed - Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355(3):260-269.
doi pubmed - Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, Khariton Y, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27(11):1954-1960.
doi pubmed - Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner-La Rocca HP, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461.
doi pubmed - Wagdy K, Nagy S. EMPEROR-Preserved: SGLT2 inhibitors breakthrough in the management of heart failure with preserved ejection fraction. Glob Cardiol Sci Pract. 2021;2021(3):e202117.
doi pubmed - Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591-602.
doi pubmed - Nair N. Epidemiology and pathogenesis of heart failure with preserved ejection fraction. Rev Cardiovasc Med. 2020;21(4):531-540.
doi pubmed - Hamam A, Abou-Omar M, Rabah H, Khattab H, Alaarag A. Worsening effect of testosterone deficiency on males with heart failure with preserved ejection fraction. BMC Endocr Disord. 2022;22(1):321.
doi pubmed - Zhao D, Guallar E, Ballantyne CM, Post WS, Ouyang P, Vaidya D, Jia X, et al. Sex hormones and incident heart failure in men and postmenopausal women: the atherosclerosis risk in communities study. J Clin Endocrinol Metab. 2020;105(10):e3798-3807.
doi pubmed - Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861-870.
doi pubmed - Coiro S, Huttin O, Kobayashi M, Lamiral Z, Simonovic D, Zannad F, Rossignol P, et al. Validation of the MEDIA echo score for the prognosis of heart failure with preserved ejection fraction. Heart Fail Rev. 2023;28(2):453-464.
doi pubmed - Rob D, Marek J, Dostalova G, Linhart A. Heart failure in Fabry disease revisited: application of current heart failure guidelines and recommendations. ESC Heart Fail. 2022;9(6):4043-4052.
doi pubmed - Hitsumoto T. Usefulness of the whole blood passage time as a predictor of primary cardiovascular events in patients with traditional cardiovascular risk factors. Cardiol Res. 2018;9(4):231-238.
doi pubmed - Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Hara S, Ura N, et al. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2007;11(1):41-50.
doi pubmed - Cesarone MR, Belcaro G, Carratelli M, Cornelli U, De Sanctis MT, Incandela L, Barsotti A, et al. A simple test to monitor oxidative stress. Int Angiol. 1999;18(2):127-130.
pubmed - Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97(12):964-969.
doi pubmed - Gazewood JD, Turner PL. Heart failure with preserved ejection fraction: diagnosis and management. Am Fam Physician. 2017;96(9):582-588.
pubmed - Obokata M, Reddy YNV, Borlaug BA. Diastolic dysfunction and heart failure with preserved ejection fraction: understanding mechanisms by using noninvasive methods. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):245-257.
doi pubmed - Jin Q, Lou Y, Chen H, Li T, Bao X, Liu Q, He X. Lower free testosterone level is correlated with left ventricular diastolic dysfunction in asymptomatic middle-aged men with type 2 diabetes mellitus. Int J Clin Pract. 2014;68(12):1454-1461.
doi pubmed - Tinetti M, Gysel M, Farias J, Ferrer M, Lombardero M, Baranchuk A. Left ventricular filling pressure in male patients with type 2 diabetes and normal versus low total testosterone levels. Cardiol J. 2015;22(2):206-211.
doi pubmed - Culic V, Busic Z. Testosterone may influence left ventricular diastolic function depending on previous myocardial infarction and smoking. Int J Cardiol. 2015;186:67-71.
doi pubmed - Setoguchi M, Hashimoto Y, Sasaoka T, Ashikaga T, Isobe M. Risk factors for rehospitalization in heart failure with preserved ejection fraction compared with reduced ejection fraction. Heart Vessels. 2015;30(5):595-603.
doi pubmed - Chen H, Chhor M, Rayner BS, McGrath K, McClements L. Evaluation of the diagnostic accuracy of current biomarkers in heart failure with preserved ejection fraction: A systematic review and meta-analysis. Arch Cardiovasc Dis. 2021;114(12):793-804.
doi pubmed - van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013;61(14):1498-1506.
doi pubmed - Hitsumoto T. Efficacy of the reactive oxygen metabolite test as a predictor of initial heart failure hospitalization in elderly patients with chronic heart failure. Cardiol Res. 2018;9(3):153-160.
doi pubmed - Watson CJ, Gallagher J, Wilkinson M, Russell-Hallinan A, Tea I, James S, O'Reilly J, et al. Biomarker profiling for risk of future heart failure (HFpEF) development. J Transl Med. 2021;19(1):61.
doi pubmed - Cong T, Jiang S, Wang K, Zhong L, Wu J, Su D. Predictive value of brachial-ankle artery pulse wave velocity to heart failure with preserved ejection fraction in hospitalised patients with acute dyspnoea. Pak J Med Sci. 2015;31(3):516-521.
doi pubmed - Hitsumoto T. Clinical significance of arterial velocity pulse index in patients with stage B heart failure with preserved ejection fraction. Cardiol Res. 2019;10(3):142-149.
doi pubmed - Schott A, Kluttig A, Mikolajczyk R, Greiser KH, Werdan K, Sedding D, Nuding S. Association of arterial stiffness and heart failure with preserved ejection fraction in the elderly population - results from the CARLA study. J Hum Hypertens. 2023;37(6):463-471.
doi pubmed - Hitsumoto T. Relationship between serum total testosterone concentration and augmentation index at radial artery in Japanese postmenopausal patients. J Clin Med Res. 2017;9(10):872-878.
doi pubmed - Shiraki N, Nakashima A, Doi S, Carrero JJ, Sugiya N, Ueno T, Stenvinkel P, et al. Low serum testosterone is associated with atherosclerosis in postmenopausal women undergoing hemodialysis. Clin Exp Nephrol. 2014;18(3):499-506.
doi pubmed - Hitsumoto T. Clinical impact of blood testosterone concentration on cardio-ankle vascular index in female patients with type 2 diabetes mellitus. Cardiol Res. 2019;10(1):9-17.
doi pubmed - Suzuki S, Motoki H, Minamisawa M, Okuma Y, Shoin W, Okano T, Kimura K, et al. Prognostic significance of high-sensitivity cardiac troponin in patients with heart failure with preserved ejection fraction. Heart Vessels. 2019;34(10):1650-1656.
doi pubmed - Gohar A, Chong JPC, Liew OW, den Ruijter H, de Kleijn DPV, Sim D, Yeo DPS, et al. The prognostic value of highly sensitive cardiac troponin assays for adverse events in men and women with stable heart failure and a preserved vs. reduced ejection fraction. Eur J Heart Fail. 2017;19(12):1638-1647.
doi pubmed - Chung CC, Hsu RC, Kao YH, Liou JP, Lu YY, Chen YJ. Androgen attenuates cardiac fibroblasts activations through modulations of transforming growth factor-beta and angiotensin II signaling. Int J Cardiol. 2014;176(2):386-393.
doi pubmed - Tsang S, Wu S, Liu J, Wong TM. Testosterone protects rat hearts against ischaemic insults by enhancing the effects of alpha(1)-adrenoceptor stimulation. Br J Pharmacol. 2008;153(4):693-709.
doi pubmed - Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119(24):3070-3077.
doi pubmed - Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(25 Pt A):2817-2827.
doi pubmed - Das DV, Saikia UK, Sarma D. Sex hormone levels - estradiol, testosterone, and sex hormone binding globulin as a risk marker for atherosclerotic coronary artery disease in post-menopausal women. Indian J Endocrinol Metab. 2019;23(1):60-66.
doi pubmed - Kaczmarek A, Reczuch K, Majda J, Banasiak W, Ponikowski P. The association of lower testosterone level with coronary artery disease in postmenopausal women. Int J Cardiol. 2003;87(1):53-57.
doi pubmed - Malkin CJ, Pugh PJ, Jones RD, Jones TH, Channer KS. Testosterone as a protective factor against atherosclerosis—immunomodulation and influence upon plaque development and stability. J Endocrinol. 2003;178(3):373-380.
doi pubmed - Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur-Farhana MF, Ima-Nirwana S, Chin KY. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22(2):129-140.
doi pubmed - van der Pol A, Gil A, Tromp J, Sillje HHW, van Veldhuisen DJ, Voors AA, Hoendermis ES, et al. OPLAH ablation leads to accumulation of 5-oxoproline, oxidative stress, fibrosis, and elevated fillings pressures: a murine model for heart failure with a preserved ejection fraction. Cardiovasc Res. 2018;114(14):1871-1882.
doi pubmed - Franssen C, Chen S, Hamdani N, Paulus WJ. From comorbidities to heart failure with preserved ejection fraction: a story of oxidative stress. Heart. 2016;102(4):320-330.
doi pubmed - Hirata Y, Yamamoto E, Tokitsu T, Kusaka H, Fujisue K, Kurokawa H, Sugamura K, et al. Reactive oxidative metabolites are associated with the severity of heart failure and predict future cardiovascular events in heart failure with preserved left ventricular ejection fraction. Int J Cardiol. 2015;179:305-308.
doi pubmed - Nishihara T, Tokitsu T, Sueta D, Oike F, Takae M, Fujisue K, Usuku H, et al. Clinical significance of reactive oxidative metabolites in patients with heart failure with reduced left ventricular ejection fraction. J Card Fail. 2021;27(1):57-66.
doi pubmed - Zhang L, Wu S, Ruan Y, Hong L, Xing X, Lai W. Testosterone suppresses oxidative stress via androgen receptor-independent pathway in murine cardiomyocytes. Mol Med Rep. 2011;4(6):1183-1188.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.