| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 4, August 2025, pages 312-320
Increase in Aortic Valve Mean Gradients One Day After Transcatheter Aortic Valve Implantation: The Role of Mitral Regurgitation
Benjamin Fogelsona, b, d, Raj Baljepallya, Eric Heidelc, Steve Ferlitaa, Travis Moodiea, Aladen Amroa, Stefan Westona
aDepartment of Medicine, University of Tennessee Graduate School of Medicine, Knoxville, TN, USA
bDivision of Cardiology, University of Tennessee Medical Center Knoxville, Knoxville, TN 37920, USA
cDepartment of Surgery, University of Tennessee Graduate School of Medicine, Knoxville, TN, USA
dCorresponding Author: Benjamin Fogelson, Division of Cardiology, University of Tennessee Medical Center Knoxville, Knoxville, TN 37920, USA
Manuscript submitted May 9, 2025, accepted June 28, 2025, published online July 28, 2025
Short title: Role of Mitral Regurgitation in Post-TAVI Gradients
doi: https://doi.org/10.14740/cr2086
| Abstract | ▴Top |
Background: Following transcatheter aortic valve implantation (TAVI), transvalvular mean gradient is known to increase from immediate to 24 h post-procedure. While anesthesia, rapid-pacing, and volume status are blamed, the true etiology is unclear. To our knowledge, no prior studies have evaluated the effects of mitral regurgitation (MR) on the rise in post-TAVI transvalvular mean gradient.
Methods: A single-center, retrospective analysis of patients who underwent TAVI at our institution between 2011 to 2020 was performed (n = 378, males = 206). Patients were divided into two groups, no-to-mild MR (n = 327) and moderate-to-severe MR (n = 51) based on echocardiograms obtained prior to TAVI. Transvalvular gradients were compared between immediate and 24-h post-TAVI echocardiograms.
Results: The average age of no-to-mild MR patients (77 years (interquartile range (IQR): 71 - 84)) was similar to moderate-to-severe MR patients (79 years (IQR: 76 - 85), p=0.13). Both groups had similar procedural blood pressures and peri-procedural medication use. The change in 24-h post-TAVI mean transvalvular gradient was +6 mm Hg (IQR: 3.7 - 9) in the moderate-to-severe MR group and +6 mm Hg (IQR: 3.4 - 9) in the no-to-mild MR group (P = 0.87).
Conclusions: In this study, we evaluated the impact of preexisting MR on changes in transvalvular gradients following TAVI. We observed no statistically significant difference in 24-h post-TAVI gradient changes between patients with moderate-to-severe MR and those with no-to-mild MR. These findings suggest that baseline MR may not be a major determinant of early post-TAVI hemodynamics; however, further prospective studies are needed to confirm this observation.
Keywords: Transcatheter aortic valve implantation; Transvalvular gradient; Mitral regurgitation
| Introduction | ▴Top |
Transthoracic echocardiogram (TTE) has an important role in transcatheter aortic valve implantation (TAVI) from pre-procedural planning to long-term post-procedural follow-up. Immediately following TAVI, TTE is used to determine post-procedural success including appropriate TAVI valve placement and function, as well as identification of paravalvular leak and/or complications [1, 2]. Obtaining mean and max transvalvular gradients are a key component to assessing TAVI function [2]. While some studies have demonstrated that elevated long-term post-TAVI gradients are associated with worse outcomes and greater mortality [3], others have found no linear correlation between mortality and high post-TAVI gradients [4, 5].
Following TAVI, there is a notable increase in mean and max transvalvular gradients when comparing immediate to 24-h post-TAVI echocardiograms. Prior studies have attributed this phenomenon to a low-flow state in the intra-procedural and immediate post-procedural setting [6]. Multiple procedural factors have been considered “low-flow state inducers” including sedation or anesthesia, rapid pacing-induced ischemia, and fasting-related hypovolemia [7]. However, to our knowledge, no prior studies have considered the impact of baseline mitral regurgitation (MR) on the 24-h transvalvular gradient change phenomenon.
The effect of concomitant MR and aortic stenosis (AS) on post-TAVI outcomes has been well presented in the current literature [8-12]. Prior studies have also demonstrated significant improvement in baseline MR with the resolution of forward flow obstruction following TAVI [12, 13]. We sought to assess whether patients with moderate-to-severe MR at the time of TAVI had a greater increase in transvalvular gradients at 24 h post-TAVI compared to patients with no-to-mild MR.
| Materials and Methods | ▴Top |
Study design
We retrospectively reviewed patients who underwent TAVI at our institution between 2011 and 2020. The study was approved by the Institutional Review Board (IRB) (IRB registration number: 00005012). Informed consent was waived by our institution’s IRB, given this is a retrospective study. The study was conducted in compliance with the ethical standards of our institution and the revised Helsinki Declaration.
Patient population
Patients with severe AS that underwent TAVI between January 2011 to August 2020 at our institution were included in this retrospective study. Patients in the study underwent a thorough, standardized evaluation by our multidisciplinary heart team prior to TAVI. All forms of AS (normal flow-low gradient, low flow-low gradient, paradoxical low flow-low gradient and classical normal flow-high gradient) were included in the study. All patients in the study received Edwards SAPIEN balloon-expandable valves [14]. Prior studies have demonstrated variations in gradients when comparing self-expanding TAVI valves and balloon-expanding valves [15, 16]. Given these known gradient discrepancies, patients that received self-expanding TAVI valves were excluded from the study. Patients with significant paravalvular leak following TAVI were excluded from the study given the potential effects of post-TAVI transvalvular gradients. Patients in the study must have had a TTE immediately and 24 h post-TAVI. Those who had TTE performed outside the 24-h window or at an outside facility were excluded from the study. Patients who underwent concomitant MV therapy were excluded. Additionally, patients with mechanical or bioprosthetic valves were excluded from the study.
As discussed, several factors have been previously considered to explain the low-flow state and transvalvular gradient changes. We considered these factors in the pre-, peri- and post-procedural settings. Pre- and post-procedural blood pressures and weight were compared between the two groups as a surrogate to volume status. Additionally, intravenous fluids received were also taken into consideration. Procedural and anesthesia documentation were reviewed to record the use of inhaled anesthetics, vasopressors, and total dosage of midazolam, propofol and fentanyl administered during TAVI.
Doppler-echocardiographic measurements - TAVI valve
All echocardiograms were interpreted in accordance with the American Society of Echocardiography (ASE) clinical recommendations. Pre-TAVI TTE was obtained to access the severity of AS. With the utilization of continuous-wave Doppler ultrasound, the peak velocity across aortic valve was measured in the apical five-chamber view and apical long axis view. Aortic valve area (AVA) was calculated using the continuity equation. The left ventricular outflow tract (LVOT) diameter was measured from the inner edge to inner edge in the parasternal long axis view with zoom. LVOT velocity was assessed using pulse-wave Doppler in the apical long axis view. Peak gradient, mean gradient and continuous wave velocity was obtained in the apical five-chamber and/or apical long axis view for immediate and 24-h post-TAVI TTEs. For patients with atrial fibrillation, an average of three beats were obtained.
Doppler-echocardiographic measurements - mitral valve (MV)
Echocardiographic assessment of MR was performed in accordance with the most current guidelines provided by the ASE at the time of analysis [17]. For consistency, all TTEs were independently re-reviewed by a study investigator. None of the original echocardiographic report data were used. MR severity was classified based on direct image reassessment using standardized quantitative and semi-quantitative parameters, including vena contracta (VC), effective regurgitant orifice area (EROA) by proximal isovelocity surface area (PISA), and regurgitant volume.
VC
VC was measured in all patients at the narrowest portion of the mitral regurgitant flow at or immediately downstream of the regurgitant orifice. Patients with VC measurements < 0.3 cm were considered to have no-to-mild MR, while patients with VC widths > 0.3 cm were considered to have moderate-to-severe MR for the purposes of this study. Great effort was placed into measuring the smallest flow area for accurate VC given that small errors can lead to misclassification of MR severity.
EROA by flow convergence method (PISA)
PISA radius measurement was performed for each patient in the study if an appropriate color frame corresponding to the maximal jet velocity was obtainable. The PISA radius was then used to calculate a PISA (2πrPISA2). The PISA was then used to calculate the regurgitant flow (Reg flow = PISA × aliasing velocity). The regurgitant flow could then be divided by the peak velocity of the regurgitant MR jet to provide the EROA (EROA = Reg flow/VMax). Patients with an EROA < 0.20 cm2 were considered no-to-mild MR, and patients with EROA > 0.20 cm2 were considered moderate-to-severe MR.
Regurgitant volume and EROA by continuity method
The MV annulus diameter and the LVOT diameter were obtained for calculation of the MV area and LVOT area, respectively (area = π (diameter/2)2). Once the area was calculated for both the MV and LVOT, the regurgitant volume was calculated using the following equation, (MV area × MV VTI) - (LVOT area × LVOT VTI). Patients with regurgitant volumes > 30 mL were considered moderate-to-severe MR. Patients with regurgitant volumes < 30 mL were put in the no-to-mild MR group. Patients with an EROA < 0.20 cm2 were considered no-to-mild MR, and patients with EROA > 0.20 cm2 were considered moderate-to-severe MR.
Primary and secondary endpoint measurements
The primary outcome assessed in this study was the numerical change (delta) in mean and max transvalvular gradients from immediate and 24 h post-TAVI echocardiograms.
Statistical analysis
The MR groups (no-to-mild vs. moderate-to-severe) were compared on baseline demographic and clinical characteristics, and echocardiographic characteristics. Chi-square or Fisher’s exact tests were used for categorical parameters, and Mann-Whitney U tests were used for continuous variables that violated normality, as determined by Shapiro-Wilk tests. Frequency and percentage statistics were used to give context to the Chi-square analyses, and medians with interquartile range (IQR) were reported for the group comparisons using Mann-Whitney U tests. Statistical significance was assumed at an alpha value of 0.05, and all analyses were performed using SPSS Version 29 (Armonk, NY: IBM Corp.).
| Results | ▴Top |
A total of 378 patients that underwent TAVI between January 2011 and December 2020 with an Edwards SAPIEN valve were included in the study. Of those, 327 patients had no-to-mild MR and 51 patients had moderate-to-severe MR (Table 1).
 Click to view | Table 1. Comparing Baseline Clinical Characteristics Between Patients With No-to-Mild MR and Patients With Moderate-to-Severe MR |
Baseline clinical characteristics
All patients received balloon-expandable Edwards SAPIEN valves in the study with the most common valve being the Edwards SAPIEN 3 valve (84.7%). The mean age of patients with no-to-mild MR was 77 years (IQR: 71 - 84) and 177 (54.1%) were males. The mean age of the moderate-to-severe MR population was 79 years (IQR: 76 - 85) and 29 (56.9%) were males. The baseline clinical data for the patients included in this study are summarized in Table 1. All baseline clinical history was similar between the no-to-mild MR and moderate-to-severe MR groups, except prior coronary artery bypass grafting (CABG), chronic kidney disease, and body mass index (BMI). Patients with moderate-to-severe MR had a greater frequency of chronic kidney disease (n = 33, 64.7%) with statistically significant lower glomerular filtration rate (GFR) of 48.5 mL/min (IQR: 32.7 - 65.1) compared to 62.9 mL/min (IQR: 43.3 - 78) in the no-to-mild MR group. Patients with moderate-to-severe MR also had a greater history of prior CABG (n = 20, 39.2%) and BMI 26.4 (IQR: 23.9 - 30) compared to patients with no-to-mild MR (n = 64, 19.6% and 29 (IQR: 25 - 34.8), respectively. Most patients in both groups had New York Heart Association (NYHA) class III-IV symptoms prior to TAVI (94.8% in the no-to-mild MR group vs. 94.1% in the moderate-to-severe MR group).
Pre-, peri-, and post-procedural characteristics
Patients with no-to-mild MR had higher pre- and post-TAVI body weights compared to those with moderate-to-severe MR (P = 0.005 and P = 0.006, respectively). However, both groups demonstrated an average weight increase of less than 0.5 kg following the procedure (no-to-mild MR P = 0.006 and moderate-to-severe MR P = 0.005). There were no significant differences between groups in terms of pre-, peri-, or post-procedural fluid administration. Additionally, pre- and post-procedural blood pressures were comparable. As shown in Table 2, the use of vasopressors, inhaled anesthetics, and peri-procedural medication dosages was similar between groups.
 Click to view | Table 2. Comparing Various Factors Considered to Be Low-Flow State Inducers Between Patients With No-to-Mild MR and Patients With Moderate-to-Severe MR |
Baseline echocardiographic characteristics, TAVI gradient delta and MR
Immediate and 24-h echocardiogram comparisons between the two groups are outlined in Table 3. Following TAVI, both groups were noted to have improvement in MR severity when comparing pre-TAVI to post-TAVI echocardiograms. The quantitative MR data for both pre- and post-TAVI assessments are summarized in Table 4. Patients in the no-to-mild MR group did not have sufficiently measurable MR jets to permit reliable quantification, and values in this cohort are therefore reported categorically as below the established thresholds for moderate MR.
 Click to view | Table 3. Comparing Echocardiographic Characteristics Between Patients With No-to-Mild MR and Patients With Moderate-to-Severe MR |
 Click to view | Table 4. Quantitative Echocardiographic Measurements of MR Before and After TAVI, Stratified by MR Severity Groups |
Additionally, both groups were noted to have a statistically significant increase in transvalvular gradients in 24 h following TAVI. However, the 24-h change in post-TAVI mean and peak transvalvular gradients did not significantly differ between patients with no-to-mild MR and patients with moderate-to-severe MR (Figs. 1, 2). Furthermore, a scatter plot of change in regurgitant volume and change in transvalvular gradient (Supplementary Material 1, cr.elmerpub.com) showed no apparent correlation between changes in MR severity and changes in mean aortic gradient following TAVI.
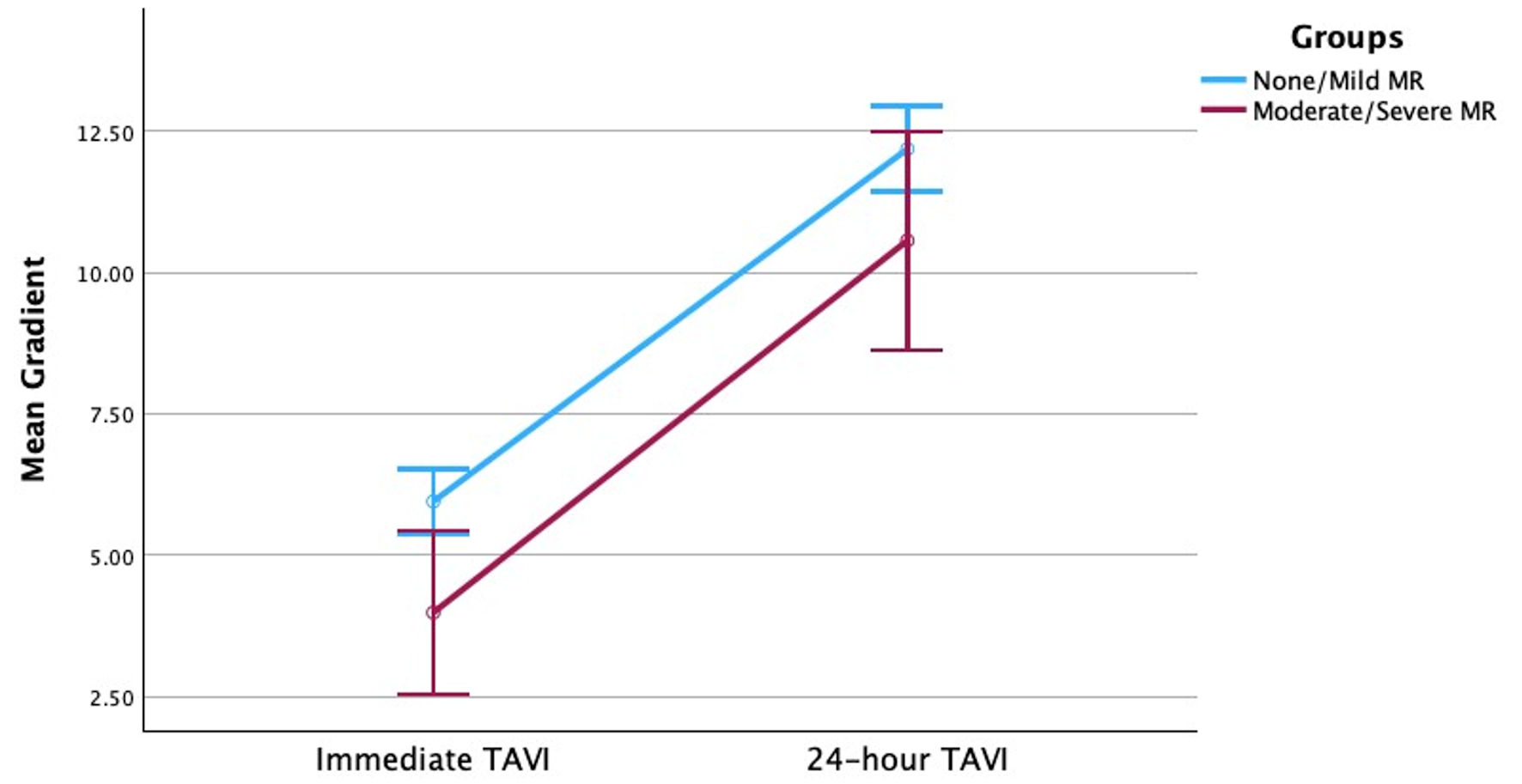 Click for large image | Figure 1. Change in transvalvular mean gradient across time for patients with no-to-mild MR and patients with moderate-to-severe MR. MR: mitral regurgitation; TAVI: transcatheter aortic valve implantation. |
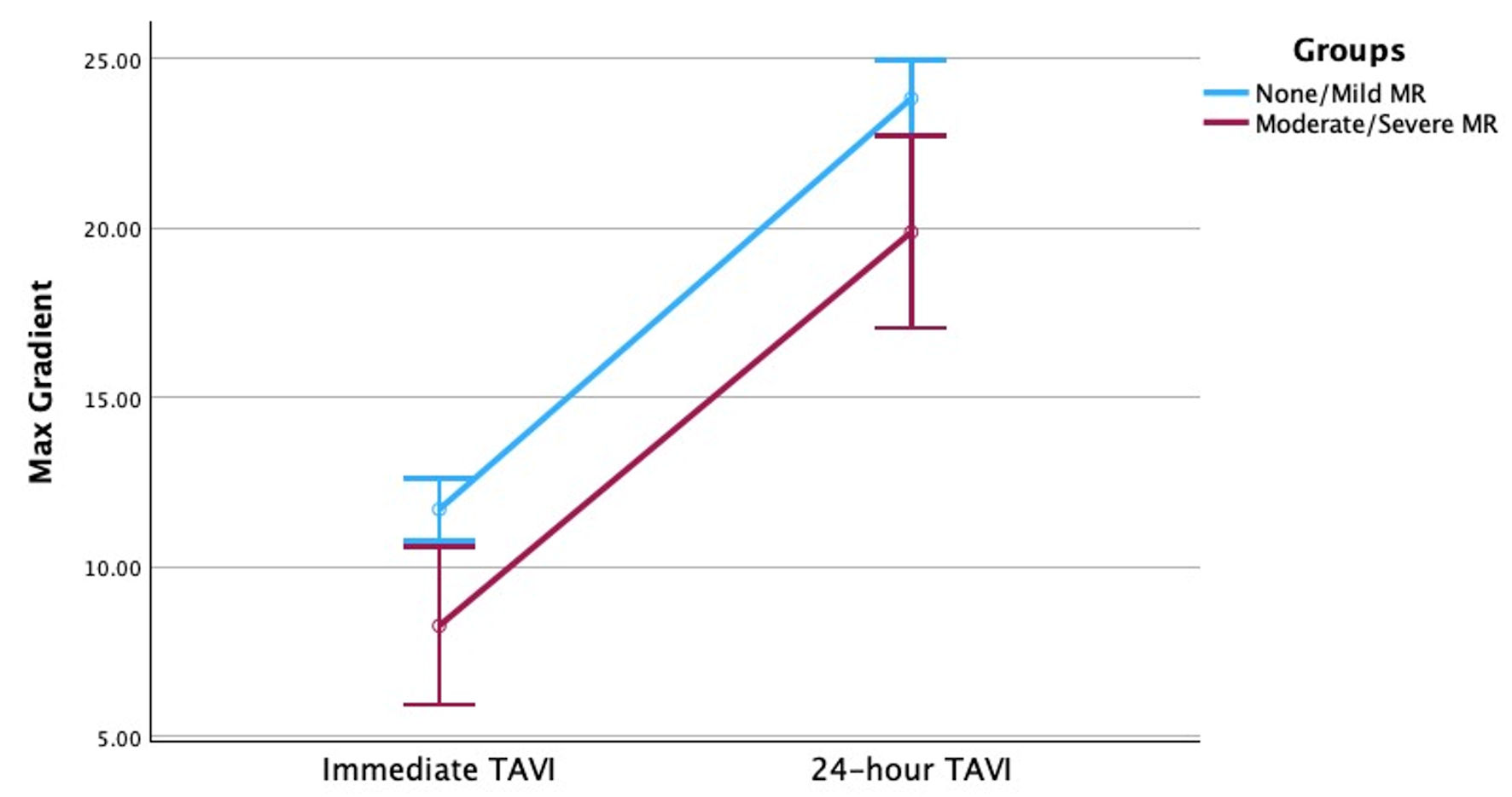 Click for large image | Figure 2. Change in transvalvular max gradient across time for patients with no-to-mild MR and patients with moderate-to-severe MR. MR: mitral regurgitation; TAVI: transcatheter aortic valve implantation. |
| Discussion | ▴Top |
In this study we compared changes in mean and peak transvalvular gradients following TAVI in patients with no-to-mild MR and moderate-to-severe MR. The main findings of this study were: 1) There was an increase in mean and max transvalvular gradients 24 h post-TAVI regardless of baseline MR severity; 2) There was a statistically significant improvement in MR severity in all patients with baseline MR following TAVI; 3) There was no significant difference in 24-h post-TAVI mean and peak transvalvular gradient deltas between patients with moderate-to-severe MR and patients with no-to-mild MR.
We considered our findings in the context of prior research evaluating 24-h post-TAVI increases in transvalvular gradients. Changes in 24-h post-TAVI transvalvular gradients are thought to be due to an induced low flow state during the procedure. Prior studies have considered medications, pre-procedural nil per os (NPO) status, and rapid-pacing transient ischemia as potential factors that may induce a low flow state [7]. The current study considered these various factors, in addition to blood pressure recordings, baseline ejection fraction, medication dosages, use of vasopressors, and administered fluids. To our knowledge, no prior studies have considered pre-existing MV disease when evaluating transvalvular gradient changes following TAVI. Our study aimed to determine the effects of pre-existing MV disease on transvalvular gradient changes following TAVI.
The observed increase in transvalvular gradients at 24 h post-TAVI may reflect early hemodynamic adaptations rather than procedural complications. While transient low-flow states immediately following TAVI have been proposed as a contributing factor, they may not fully account for the magnitude of change. Prior work by Naidu et al [7] similarly reported an increase in transvalvular gradients from immediate to early post-TAVI follow-up, noting that this rise was not proportional to changes in stroke volume. Their findings support the notion that both low-flow physiology and evolving post-procedural ventricular loading conditions may influence early gradient measurements.
While prior studies have focused on procedural factors that induce low-flow states during TAVI, few have evaluated the effects of pre-existing MV disease on transvalvular gradient changes following the procedure. Concomitant MR and severe AS are frequently encountered in patients undergoing TAVI [18, 19]. In the setting of severe AS, functional MR (FMR) is more commonly observed than primary (degenerative) MR [20]. Prior studies have suggested that MR may be a significant hemodynamic determinant of a low-flow state in this population [21]. This theory is supported by data showing that moderate-to-severe MR can lead to underestimation of AS severity [18]. It has been proposed that FMR in the context of AS may arise from direct aortic valve obstruction and elevated left ventricular (LV) pressure [22]. However, more recent literature indicates that FMR is more likely related to impaired longitudinal LV function and adverse ventricular remodeling [20].
These pathophysiologic mechanisms were considered in the present study. Among patients with moderate-to-severe MR, we assessed the etiology and found that 49 of 51 patients (96%) had degenerative MR, while only two patients (4%) had FMR. Due to the minimal MR burden in the no-to-mild MR group, etiology was not further classified in that cohort. The predominance of degenerative MR in our population may help explain the absence of a significant difference in post-TAVI transvalvular gradient changes between groups, in contrast to prior studies that focused on FMR and its potential contribution to low-flow physiology.
Although MR severity may improve rapidly following TAVI [12, 13, 23], we hypothesized that the decrease in LV pressure and recovery of ventricular function and remodeling do not occur immediately. Rather, transvalvular gradient changes are likely to evolve over the early post-TAVI period. Based on this hypothesis, we expected that patients with moderate-to-severe MR would exhibit a more pronounced increase in transvalvular gradients at 24 h post-TAVI compared to those with no-to-mild MR. However, our findings demonstrated no statistically significant difference in 24-h mean or peak transvalvular gradient changes between the two groups. While we found no significant difference in the 24-h transvalvular gradient changes between the two groups, the measurable effects of TAVI on LV function, LV remodeling, and MR may take more than 24 hours. In the study performed by Naidu et al [7], they demonstrated that transvalvular gradients were high at day 1 and at day 30 post TAVI when compared to immediate post-TAVI transvalvular gradients. Following TAVI, the improvement in MR may not demonstrate significant effects on transvalvular gradients for multiple days to weeks post-TAVI as the left ventricle undergoes progressive remodeling. More studies are needed to determine the effects of baseline MR on the change in transvalvular gradients in the short-term and long-term post-TAVI setting.
Another important consideration in patients with severe AS and elevated left ventricular end diastolic pressure (LVEDP) is the effects of microvascular dysfunction. Studies have shown that microvascular remodeling is frequently found in patients with severe AS and elevated LVEDP [24-26]. Ischemic MR should be considered in patients with microvascular dysfunction in the setting of AS and elevated LVEDP. The reduction in LVEDP and improvement in microvascular function following TAVI is likely observed indirectly by the post-procedural improvement in MR severity. While there is a measurable improvement in MR severity immediately post TAVI, the impact of microvascular dysfunction on post-TAVI transvalvular gradients remains unclear.
The current study’s limitations are discussed below. The results of this single-center study should be interpreted considering its smaller sample size and its retrospective nature. Although retrospective in nature, this study utilized uniform re-analysis of all echocardiograms using current ASE guidelines to mitigate inconsistencies related to evolving measurement criteria over the study period. Patients included in this study only received Edwards SAPIEN balloon-expandable valves and therefore the results should not be applied to self-expanding TAVI valves. In the current study, MR valve measurements and MR severity grading were performed by an independent reader who was not blinded, which could present potential bias. Most patients included in the study were suspected to have FMR in the setting of severe AS, however the etiology of the MR was not differentiated. As previously discussed, immediate and 24-h post-TAVI echocardiograms were reviewed and therefore the effects of MR on transvalvular gradients after this period were not accounted for in the study. More studies are needed to evaluate the etiology behind the increase in post-TAVI transvalvular gradients and the effects of baseline concomitant valvular disease on transvalvular gradients.
Conclusions
In this retrospective analysis, we directly evaluated the role of MR in early post-TAVI transvalvular gradient changes and found that moderate-to-severe MR did not significantly influence the 24-h gradient increase compared to no-to-mild MR. Despite prior hypotheses suggesting a potential role for MR as a contributor to low-flow states and gradient dynamics following TAVI, our findings indicate that baseline MR severity does not appear to independently affect early post-TAVI transvalvular hemodynamics. These results suggest that other factors, such as intraprocedural hemodynamics, myocardial remodeling, and microvascular function, may play more prominent roles in the observed gradient changes. Future prospective studies with longer-term follow-up are warranted to further elucidate the interaction between baseline MR, LV remodeling, and transvalvular gradients in the post-TAVI period.
| Supplementary Material | ▴Top |
Suppl 1. Scatter plot depicting the relationship between the change in MR severity - quantified by regurgitant volume (ΔRV) - and the change in mean aortic transvalvular gradient (ΔGradient) from immediately post-TAVI to 24 h post-TAVI. Each point represents an individual patient with moderate-to-severe MR. Visual assessment using quantitative MR data demonstrates no clear correlation between ΔRV and ΔGradient, suggesting that early post-procedural changes in transvalvular gradients are not significantly influenced by concurrent MR severity.
Acknowledgments
We would like to express our gratitude to all those who helped us during the writing of this manuscript. We would also like to thank all the peer reviewers for their suggestions.
Financial Disclosure
None to declare.
Conflict of Interest
The authors report no conflict of interest regarding the content herein.
Informed Consent
Informed consent was waived by our institution’s IRB, as this is a retrospective study.
Author Contributions
Benjamin Fogelson: methodology, conceptualization, investigation, writing - original draft, review and editing. Raj Baljepally: methodology, conceptualization, writing, review, editing, and supervision. Eric Heidel: biostatistics, formal analysis, writing, review and editing. Steve Ferlita: investigation, validation. Travis Moodie: investigation, validation. Aladen Amro: investigation, validation, Stefan Weston: investigation, validation.
Data Availability
The data supporting these findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Kronzon I, Jelnin V, Ruiz CE, Saric M, Williams MR, Kasel AM, Shivaraju A, et al. Optimal imaging for guiding TAVR: transesophageal or transthoracic echocardiography, or just fluoroscopy? JACC Cardiovasc Imaging. 2015;8(3):361-370.
doi pubmed - Saric M, Williams MR. Transthoracic echocardiography guidance for TAVR. JACC Cardiovasc Imaging. 2015;8(3):363-367.
- Didier R, Benic C, Nasr B, Le Ven F, Hannachi S, Eltchaninoff H, Koifman E, et al. High post-procedural transvalvular gradient or delayed mean gradient increase after transcatheter aortic valve implantation: incidence, prognosis and associated variables. The FRANCE-2 Registry. J Clin Med. 2021;10(15):3221.
doi pubmed - Khalili H, Pibarot P, Hahn RT, Elmariah S, Pilgrim T, Bavry AA, Maini B, et al. Transvalvular pressure gradients and all-cause mortality following TAVR: a multicenter echocardiographic and invasive registry. JACC Cardiovasc Interv. 2022;15(18):1837-1848.
doi pubmed - Thyregod HGH, Ihlemann N. Measuring transvalvular aortic pressure gradients: answering questions or asking new ones? JACC Cardiovasc Interv. 2022;15(18):1849-1851.
doi pubmed - Stanova V, Rieu R, Cote N, Salaun E, Rodes-Cabau J, Pibarot P. In vitro Doppler versus catheter transvalvular pressure gradients in balloon-expandable versus self-expanding transcatheter aortic valves. Catheter Cardiovasc Interv. 2022;99(1):201-210.
doi pubmed - Naidu S, Chen T, Fiorilli P, Li RH, Desai N, Szeto WY, Giri J, et al. Measuring TAVR prosthesis gradient immediately post-procedure may underestimate its significance. JACC Cardiovasc Interv. 2022;15(1):120-121.
doi pubmed - Nappi F, Nenna A, Timofeeva I, Mihos C, Gentile F, Chello M. Mitral regurgitation after transcatheter aortic valve replacement. J Thorac Dis. 2020;12(5):2926-2935.
doi pubmed - Stahli BE, Reinthaler M, Leistner DM, Landmesser U, Lauten A. Transcatheter aortic valve replacement and concomitant mitral regurgitation. Front Cardiovasc Med. 2018;5:74.
doi pubmed - Kindya B, Ouzan E, Lerakis S, Gonen E, Babaliaros V, Karayel E, Thourani VH, et al. Degenerative mitral regurgitation predicts worse outcomes in patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2018;92(3):574-582.
doi pubmed - Witberg G, Codner P, Landes U, Schwartzenberg S, Barbanti M, Valvo R, De Backer O, et al. Effect of transcatheter aortic valve replacement on concomitant mitral regurgitation and its impact on mortality. JACC Cardiovasc Interv. 2021;14(11):1181-1192.
doi pubmed - Feldt K, De Palma R, Bjursten H, Petursson P, Nielsen NE, Kellerth T, Jonsson A, et al. Change in mitral regurgitation severity impacts survival after transcatheter aortic valve replacement. Int J Cardiol. 2019;294:32-36.
doi pubmed - Chiche O, Rodes-Cabau J, Campelo-Parada F, Freitas-Ferraz AB, Regueiro A, Chamandi C, Rodriguez-Gabella T, et al. Significant mitral regurgitation in patients undergoing TAVR: Mechanisms and imaging variables associated with improvement. Echocardiography. 2019;36(4):722-731.
doi pubmed - Edwards Lifesciences. TAVR by Edwards [Internet]. Irvine (CA): Edwards Lifesciences; n.d. [cited Jun 19, 2025]. Available from: https://treatheartvalvefailure.com/about-treatment/tavr-by-edwards.
- Leone PP, Regazzoli D, Pagnesi M, Sanz-Sanchez J, Chiarito M, Cannata F, Van Mieghem NM, et al. Predictors and clinical impact of prosthesis-patient mismatch after self-expandable TAVR in small annuli. JACC Cardiovasc Interv. 2021;14(11):1218-1228.
doi pubmed - Okuno T, Tomii D, Lanz J, Heg D, Praz F, Stortecky S, Reineke D, et al. 5-year outcomes with self-expanding vs balloon-expandable transcatheter aortic valve replacement in patients with small annuli. JACC Cardiovasc Interv. 2023;16(4):429-440.
doi pubmed - American Society of Echocardiography. Guidelines and standards: 2017 ASE guideline for the evaluation of valvular regurgitation [Internet]. Durham (NC): ASE; 2017 [cited Jun 19, 2025]. Available from: https://www.asecho.org/wp-content/uploads/2017/04/2017VavularRegurgitationGuideline.pdf.
- Mantovani F, Barbieri A, Albini A, Bonini N, Fanti D, Fezzi S, Setti M, et al. The common combination of aortic stenosis with mitral regurgitation: diagnostic insight and therapeutic implications in the modern era of advanced echocardiography and percutaneous intervention. J Clin Med. 2021;10(19):4364.
doi pubmed - Ferruzzi GJ, Silverio A, Giordano A, Corcione N, Bellino M, Attisano T, Baldi C, et al. Prognostic impact of mitral regurgitation before and after transcatheter aortic valve replacement in patients with severe low-flow, low-gradient aortic stenosis. J Am Heart Assoc. 2023;12(17):e029553.
doi pubmed - Rossi A, Dandale R, Nistri S, Faggiano P, Cicoira M, Benfari G, Onorati F, et al. Functional mitral regurgitation in patients with aortic stenosis: prevalence, clinical correlates and pathophysiological determinants: a quantitative prospective study. Eur Heart J Cardiovasc Imaging. 2014;15(6):631-636.
doi pubmed - Benfari G, Clavel MA, Nistri S, Maffeis C, Vassanelli C, Enriquez-Sarano M, Rossi A. Concomitant mitral regurgitation and aortic stenosis: one step further to low-flow preserved ejection fraction aortic stenosis. Eur Heart J Cardiovasc Imaging. 2018;19(5):569-573.
doi pubmed - Sannino A, Grayburn PA. Mitral regurgitation in patients with severe aortic stenosis: diagnosis and management. Heart. 2018;104(1):16-22.
doi pubmed - Cortes C, Amat-Santos IJ, Nombela-Franco L, Munoz-Garcia AJ, Gutierrez-Ibanes E, De La Torre Hernandez JM, Cordoba-Soriano JG, et al. Mitral regurgitation after transcatheter aortic valve replacement: prognosis, imaging predictors, and potential management. JACC Cardiovasc Interv. 2016;9(15):1603-1614.
doi pubmed - Martinez Pereyra V, Seitz A, Mahrholdt H, Bekeredjian R, Sechtem U, Ong P. Coronary microvascular dysfunction in patients with mild-to-moderate aortic stenosis - Insights from intracoronary acetylcholine testing. Int J Cardiol Heart Vasc. 2020;31:100658.
doi pubmed - Julius BK, Spillmann M, Vassalli G, Villari B, Eberli FR, Hess OM. Angina pectoris in patients with aortic stenosis and normal coronary arteries. Mechanisms and pathophysiological concepts. Circulation. 1997;95(4):892-898.
doi pubmed - Rajappan K, Rimoldi OE, Dutka DP, Ariff B, Pennell DJ, Sheridan DJ, Camici PG. Mechanisms of coronary microcirculatory dysfunction in patients with aortic stenosis and angiographically normal coronary arteries. Circulation. 2002;105(4):470-476.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.