| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Case Report
Volume 16, Number 5, October 2025, pages 457-461
A Rare Case of Cardiac Myxoma With Multiple Feeding Vessels From the Right Coronary Artery and the Left Circumflex Artery
Tomo Komakia, b, c, e, Natsuki Onishib, Kohei Tashiroa, b, Yuko Teratanid, Yuta Sukehirod, Hideichi Wadad, Shin-ichiro Miurab, Masahiro Ogawaa, b, c
aThe Cardiac Arrhythmia Center and EP Laboratory, Fukuoka University Hospital, Fukuoka, Japan
bDepartment of Cardiology, Fukuoka University Hospital, Fukuoka, Japan
cDepartment of Clinical Laboratory Medicine, Fukuoka University Faculty of Medicine, Fukuoka, Japan
dDepartment of Cardiovascular Surgery, Fukuoka University Hospital, Fukuoka, Japan
eCorresponding Author: Tomo Komaki, Department of Clinical Laboratory Medicine, Fukuoka University Faculty of Medicine, Jonan-ku, Fukuoka 814-0180, Japan
Manuscript submitted July 25, 2025, accepted August 7, 2025, published online October 10, 2025
Short title: Cardiac Myxoma With Multiple Feeding Vessels
doi: https://doi.org/10.14740/cr2113
| Abstract | ▴Top |
An 80-year-old woman with persistent atrial fibrillation was referred to our hospital for evaluation of a left atrial mass. Transthoracic and transesophageal echocardiography revealed a well-defined, sessile, and immobile mass attached to the interatrial septum. Computed tomography (CT) coronary angiography revealed a cardiac tumor fed by two vessels: one from the right coronary artery and one from the left circumflex artery. Based on these findings and cardiac magnetic resonance imaging, the mass was diagnosed as a left atrial myxoma, and excision was performed. Although some atrial myxomas are highly vascular, identification of multiple feeding vessels on CT coronary angiography is rare. Preoperative evaluation of feeding vessels is helpful in distinguishing myxomas from left atrial thrombi, especially in patients with hypercoagulability.
Keywords: Myxoma; Neovascularity; Computed tomography coronary angiography; Left atrial thrombus
| Introduction | ▴Top |
Myxomas are the most common primary cardiac neoplasms. Myxomas can occur in any cardiac chamber but have a predilection for the left atrium [1]. In general, left atrial myxomas are attached to the interatrial septum, pedunculated, and highly mobile, resulting in left ventricular inflow tract obstruction. Because myxomas are friable and release various cytokines, they can also cause systemic embolization and constitutional symptoms similar to those of connective tissue diseases. In rare cases, myxomas are sessile and immobile and may be asymptomatic. In such atypical cases, it is important to differentiate them from left atrial thrombi, especially in patients with hypercoagulable conditions such as atrial fibrillation (AF), congestive heart failure, or nephrotic syndrome. Here, we report the case of an 80-year-old woman with persistent AF of unknown duration, in whom a left atrial mass attached to the interatrial septum was incidentally identified by echocardiography. The mass was sessile and immobile, and the patient had few subjective symptoms. These atypical findings made it necessary to differentiate the myxoma from left atrial thrombus before surgery.
| Case Report | ▴Top |
An 80-year-old woman had been experiencing occasional palpitations at night for 6 months. She had no previous medical history. During lower gastrointestinal endoscopy at a nearby clinic, AF was noted on the electrocardiogram. Transthoracic echocardiography revealed a left atrial mass, and she was referred to our hospital for further evaluation.
At consultation, her blood pressure was 105/77 mm Hg, pulse was irregular at 83 beats/min, temperature was 36.2 °C, and oxygen saturation on room air was 98%. She was taking 5 mg of oral apixaban daily. Her heart and lung sounds were normal, and no jugular venous distension or leg edema was detected. A 12-lead electrocardiogram showed AF with a heart rate of 110 beats/min. A chest radiograph showed cardiomegaly, with a cardiothoracic ratio of 57%.
Blood tests showed a white blood cell count of 5,100 cells/µL with no differential abnormalities and a hemoglobin level of 13.2 g/dL (normal: 11.6 - 14.8 g/dL). Liver and renal function were within normal range, but C-reactive protein was slightly elevated at 0.93 mg/dL (normal: 0 - 0.14 mg/dL), and erythrocyte sedimentation rate was also elevated at 68 mm/h (normal: 3 - 15 mm/h). Brain natriuretic peptide level was elevated at 228 pg/mL (normal: ≤ 18.4 pg/mL).
Transthoracic echocardiography showed a large (37 × 39 mm), well-defined, oval-shaped, heterogenous mass in a dilated left atrium (45 mm) (Fig. 1a). Transesophageal echocardiography confirmed that the mass was sessile and immobile with a wide septal attachment (Fig. 1b). Moderate mitral regurgitation associated with annular dilatation was observed (Fig. 1c). No mass was found in the left atrial appendage, and head magnetic resonance imaging showed no embolization.
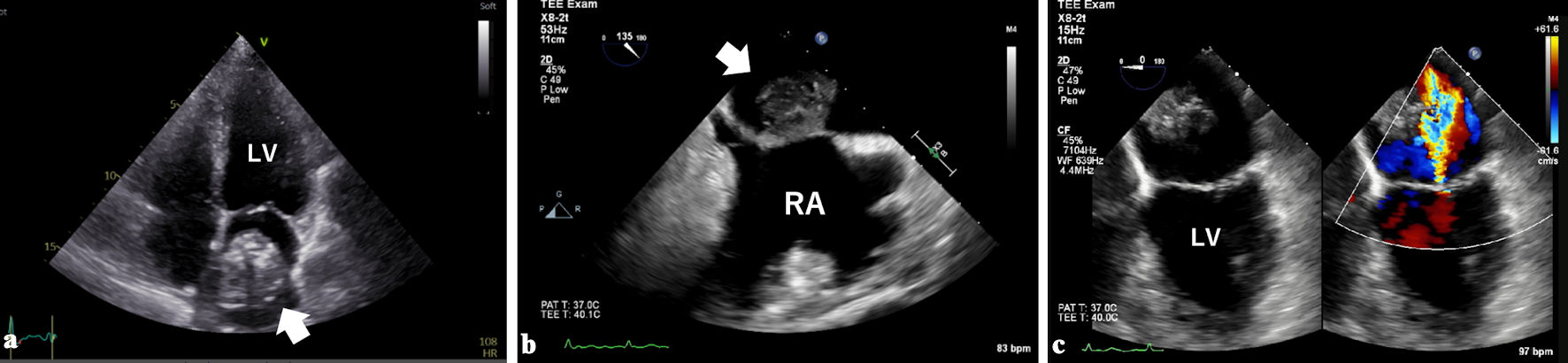 Click for large image | Figure 1. Transthoracic (a) and transesophageal echocardiography (b) showed a large mass (white arrows) in a dilated left atrium. The mass was attached to the interatrial septum with a wide base. Moderate mitral regurgitation associated with annular dilatation was observed (c). LV: left ventricle; RA: right atrium. |
Chest computed tomography (CT) showed that the mass was heterogeneously enhanced by contrast material with partial calcification (Fig. 2a). The CT coronary angiography revealed two feeding vessels: one originating from the sinus node artery of the right coronary artery (RCA) and another from the atrial branch of the left circumflex artery (LCX) (Fig. 2b, c). The presence of feeding vessels ruled out the possibility of thrombus and suggested a cardiac tumor.
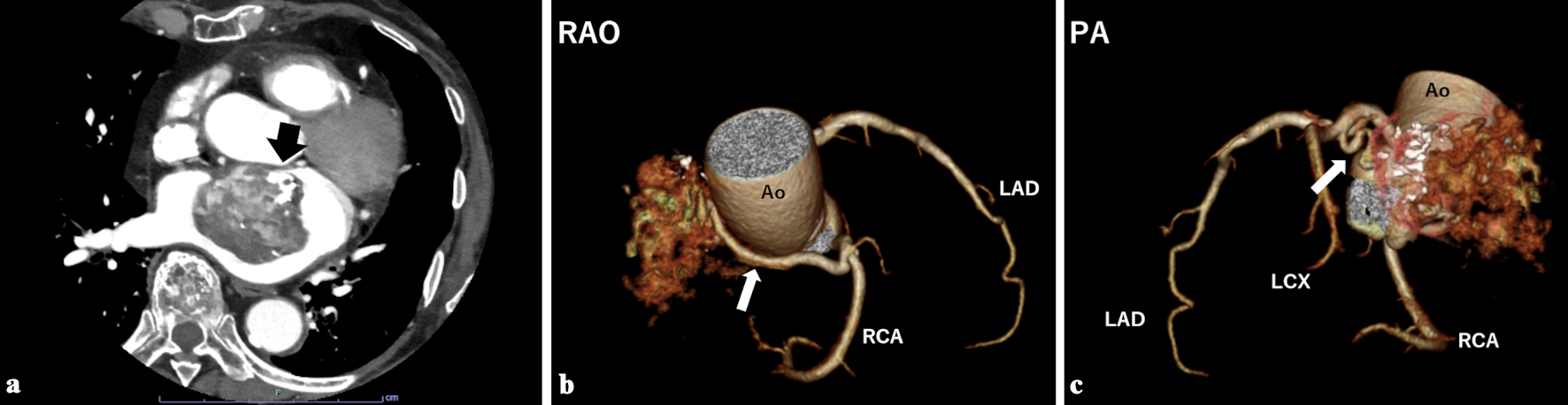 Click for large image | Figure 2. (a) Contrast-enhanced chest computed tomography showed a heterogeneously enhanced mass with partial calcification (black arrow). Computed tomography coronary angiography showed two feeding vessels originating from the right coronary artery (b, white arrow) and the left circumflex artery (c, white arrow). Ao: aorta; LAD: left anterior descending artery; LCX: left circumflex artery; PA: posterior-anterior projection; RAO: right anterior oblique projection; RCA: right coronary artery. |
Subsequently, cardiac magnetic resonance (CMR) imaging was performed for histological discrimination of the tumor. The interior of the mass was hypointense on T1-weighted (T1w) and T2-weighted (T2w) imaging, and the exterior was hyperintense on T2w imaging (Fig. 3a, b). No change was observed on fat suppression imaging. Late gadolinium enhancement (LGE) imaging showed heterogeneous enhancement (Fig. 3c). These findings were consistent with myxoma.
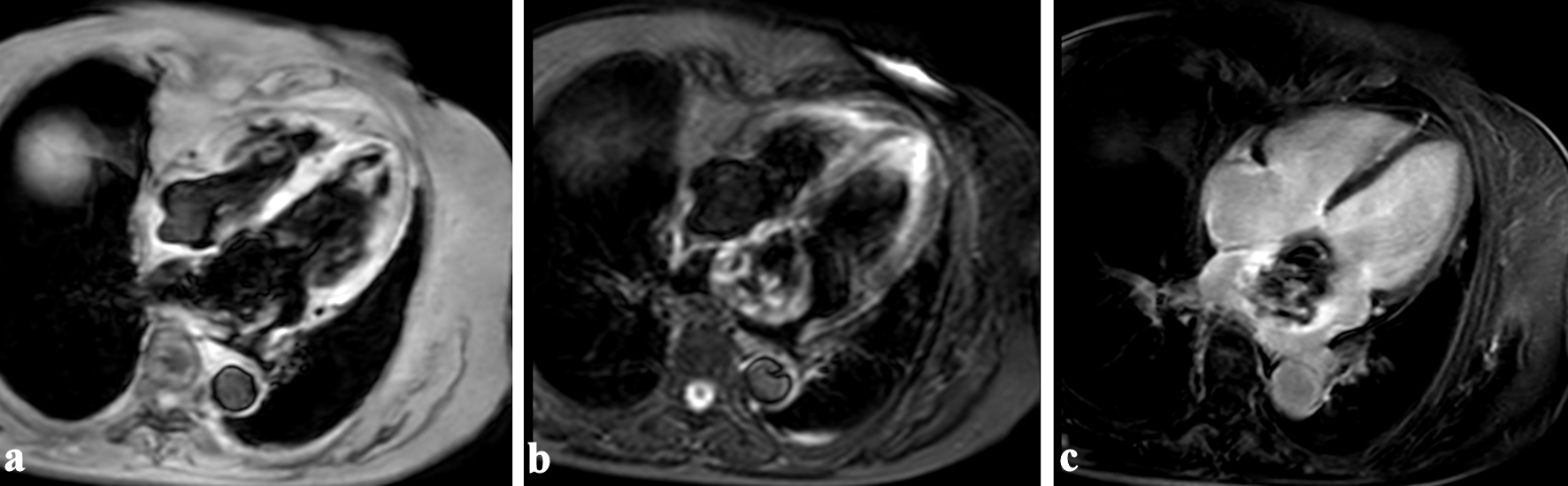 Click for large image | Figure 3. Cardiac magnetic resonance imaging showed that the interior of the mass was hypointense on T1-weighted (a) and T2-weighted imaging (b), and the exterior was hyperintense on T2-weighted imaging. Heterogeneous enhancement was observed on late gadolinium enhancement imaging (c). |
Surgery was performed via median sternotomy using cardiopulmonary bypass with antegrade blood cardioplegia. Using a trans-septal approach through right atriotomy, the tumor was completely excised. Mitral valve plasty was performed with a Physio II ring (Edwards Lifesciences Corp., Irvine, CA, USA), and pulmonary vein isolation was performed with the AtriCure bipolar radiofrequency system (AtriCure, Inc., Mason, OH, USA). The septal defect was closed with a bovine pericardial patch.
The excised tumor was reddish brown and friable and measured 50 × 45 × 40 mm (Fig. 4a). Histopathological examination showed that proliferated cells with spindle-shaped nuclei were embedded in a myxoid matrix, confirming the diagnosis of myxoma (Fig. 4b). The tumor was also characterized by hemorrhage, infiltration by lymphocytes and siderophages, degenerated elastic fibers, and partial calcification and ossification (Fig. 4c, d).
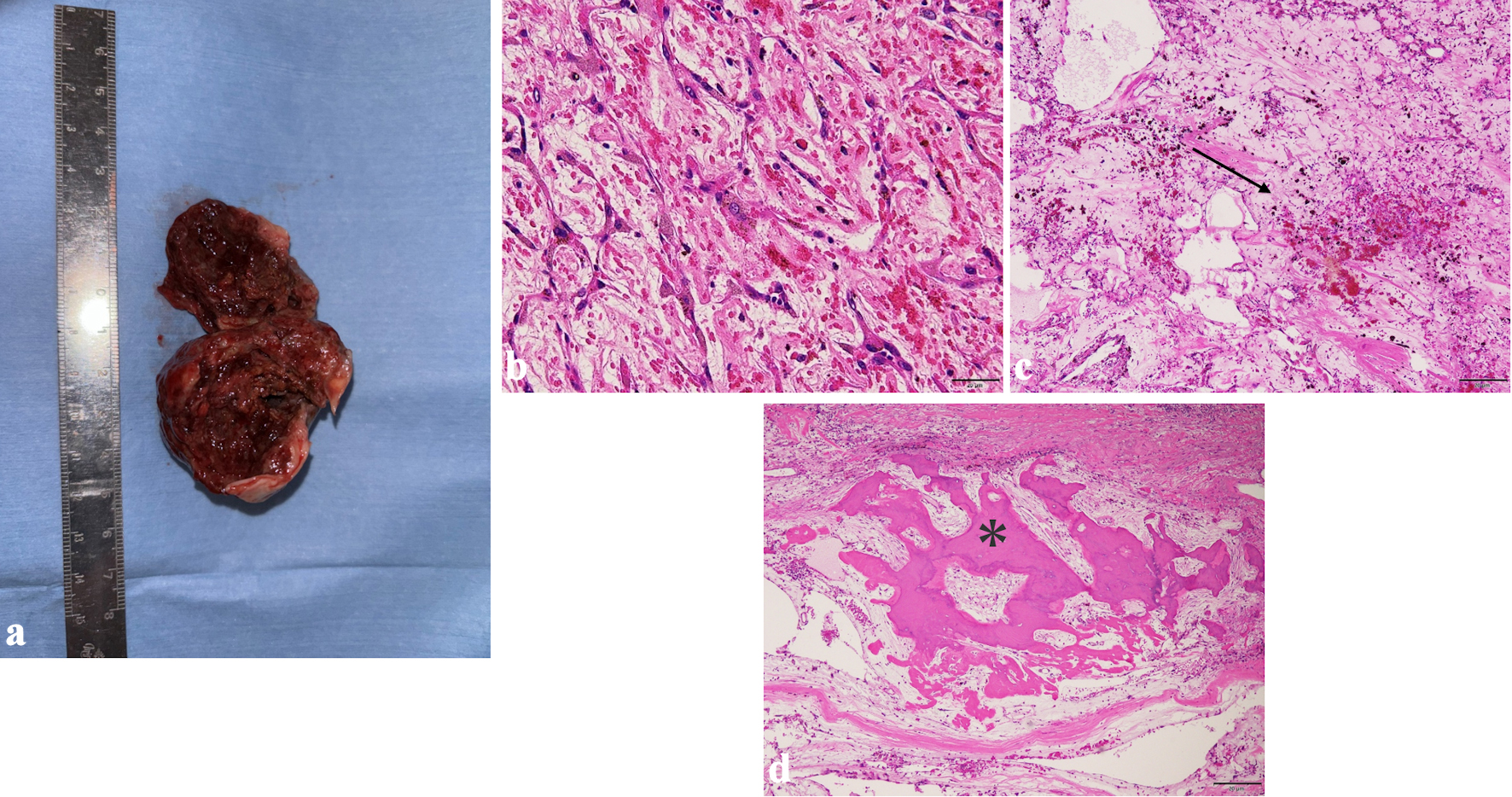 Click for large image | Figure 4. The excised tumor was reddish brown and friable and measured 50 × 45 × 40 mm (a). Photomicrographs of hematoxylin and eosin (H&E) staining sections are shown: (b) H&E staining × 200; (c) H&E staining × 40; (d) H&E staining × 40. Proliferated cells with spindle-shaped nuclei were embedded in a myxoid matrix (b). The interior of the tumor contained hemorrhage (c, black arrow) and foci of ossification (d, asterisk). |
The postoperative course was uneventful, and the patient was discharged 24 days after surgery. Follow-up CT coronary angiography at 6 months showed regression of the feeding vessels and no recurrence of the tumor (Fig. 5).
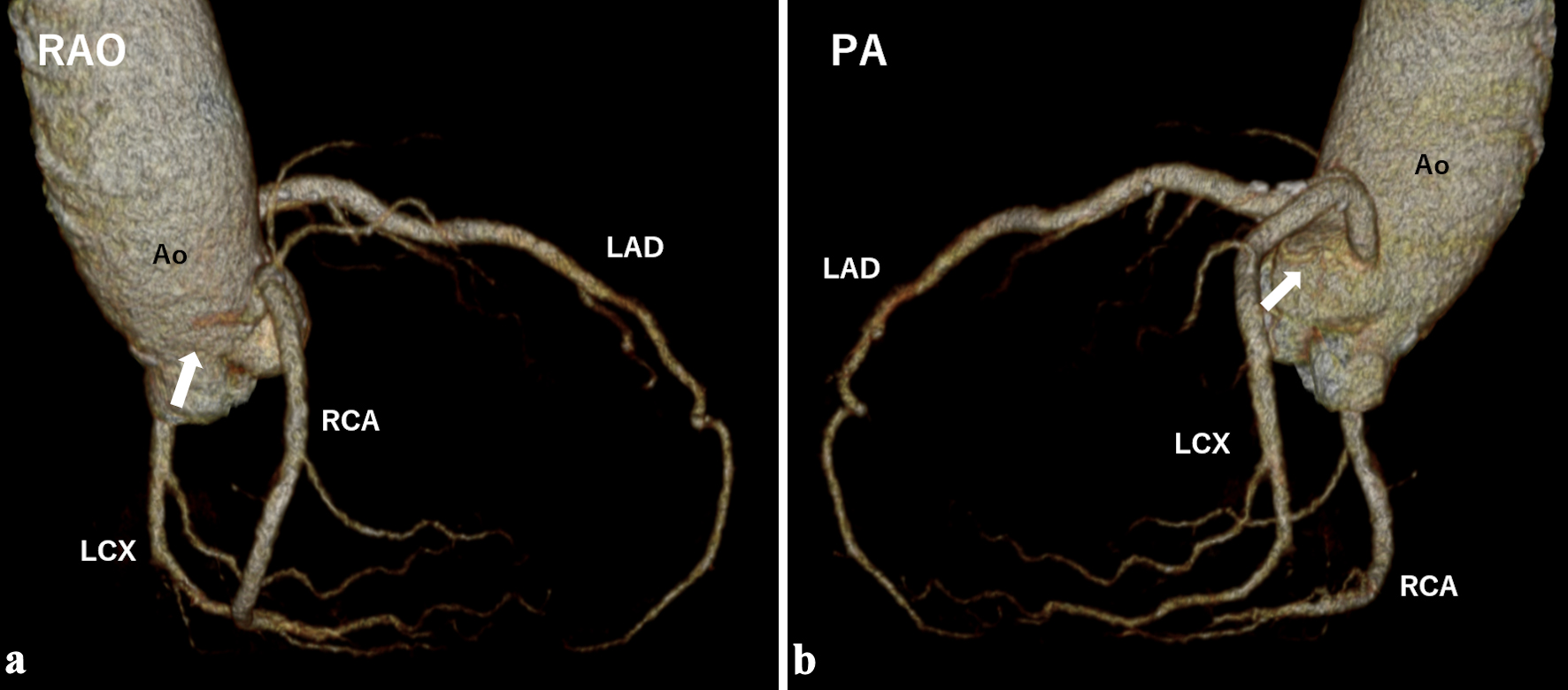 Click for large image | Figure 5. Computed tomography coronary angiography performed 6 months postoperatively showed disappearance of the feeding vessels (white arrows). Ao: aorta; LAD: left anterior descending artery; LCX: left circumflex artery; PA: posterior-anterior projection; RAO: right anterior oblique projection; RCA: right coronary artery. |
| Discussion | ▴Top |
Myxomas are benign tumors that arise from multipotent mesenchymal cells of endocardial origin, which are capable of neural and endothelial differentiation [2]. Myxomas produce vascular endothelial growth factor, which contributes to tumor growth and angiogenesis [3, 4]. They also release various cytokines such as interleukin-6, which contribute to constitutional symptoms such as fever and weight loss [5].
Left atrial myxomas are typically highly mobile with a peduncle attached to the interatrial septum, resulting in left ventricular inflow tract obstruction. As they are friable and may shed fragments, they can also cause systemic embolization. However, some atypical cases are sessile and immobile and may be asymptomatic. The period from myxoma development to clinical detection varies from case to case, and older tumors may exhibit fibrosis and calcification. Therefore, tumor components can vary depending on histologic differentiation and maturity.
Exclusion of left atrial thrombus is important in the differential diagnosis of left atrial myxoma, as treatment strategies differ: myxomas require surgical resection, and thrombi are treated with anticoagulant therapy. History of cardiac and/or constitutional symptoms supports the diagnosis of myxoma, whereas the presence of any hypercoagulability (e.g., AF, extensive anterior myocardial infarction, dilated cardiomyopathy, valvular disease) supports that of thrombus. On echocardiography, myxomas are generally attached to the interatrial septum, pedunculated, and highly mobile. In contrast, thrombi are often attached to the left atrial appendage, though they may also be attached to the interatrial septum, particularly the left atrial septal pouch [6].
In the present case, the time of onset of AF was unknown, and the left atrial mass was detected shortly after administration of anticoagulant. Because the classic myxoma triad (obstructive cardiac signs, embolic signs, and constitutional manifestations) was absent and the echocardiographic findings were atypical, both myxoma and thrombus were considered in the differential diagnosis. Because the patient had characteristics supporting both entities, differentiation based on medical history and echocardiographic findings was difficult.
Zhang et al reported that CT coronary angiography can help differentiate myxomas from thrombi based on the enhancement pattern and the presence of feeding vessels [7]. Specifically, myxomas often show mild to moderate patchy enhancement, whereas thrombi show no enhancement or only peripheral enhancement. Additionally, 83% (n = 15/18) of myxomas had feeding vessels from the coronary arteries, most commonly from the RCA (66%, 10/18), followed by the LCX (26%, 4/18) and both the RCA and LCX (6%, 1/18). In contrast, no feeding vessels were found in any of the thrombus cases. In the present case, the left atrial mass showed heterogeneous enhancement and was fed by two vessels from both the RCA and LCX. Although some atrial myxomas are highly vascular [8], the identification of multiple feeding vessels on CT coronary angiography is rare. This tumor appeared even more strongly enhanced than those previously reported, likely due to its rich blood supply.
CMR imaging is useful in the diagnosis of cardiac tumors, as it provides superior tissue contrast to other imaging modalities. Because the tissue components of myxomas vary, several imaging patterns have been reported [9]. The most common presentation is an isointense signal on T1w and a hyperintense signal on T2w imaging. In about half of patients, LGE imaging shows heterogeneous enhancement. A hyperintense signal on T2w images corresponds to the myxoid matrix, whereas foci of hypointense signal on T2w images indicate the presence of hemorrhage, hemosiderin, or calcification. Heterogeneous enhancement on LGE imaging reflects fibrous components of the tumor.
In this case, the tumor margin was rich in myxoid matrix, while the interior showed hemorrhage, degenerated elastic fibers, and partial calcification and ossification. Because fibrosis and calcification are considered degenerative phenomena [5], this myxoma had likely developed long before detection. The preoperative CMR findings were largely consistent with subsequent histopathological findings, suggesting that CMR imaging can provide accurate information. Thus, the combination of CT coronary angiography and CMR findings can allow for more reliable preoperative diagnosis of myxoma.
In the postoperative period, fistulas between feeding vessels and the cardiac chamber through the surgical incision line have been reported [10, 11]. Because fistulas can lead to distal myocardial ischemia due to the steal phenomenon and pulmonary hypertension due to shunting, coil embolization or surgical resection may be required. In this patient, we confirmed the disappearance of the feeding vessels by CT coronary angiography at 6 months postoperatively, suggesting that they thrombosed and regressed after tumor excision. Confirming the disappearance of feeding vessels, in addition to assessing for tumor recurrence, is important in the postoperative period.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
TK, NO, KT, YT, YS, HW, SM, and MO contributed to the study design, drafting, editing, and final approval of the manuscript.
Data Availability
The authors declare that the data supporting the findings of this study are available in this article.
Abbreviations
AF: atrial fibrillation; Ao: aorta; CMR: cardiac magnetic resonance; CT: computed tomography; H&E: hematoxylin and eosin; LAD: left anterior descending artery; LCX: left circumflex artery; LGE: late gadolinium enhancement; LV: left ventricle; PA: posterior-anterior projection; RA: right atrium; RAO: right anterior oblique projection; RCA: right coronary artery; T1w: T1-weighted; T2w: T2-weighted
| References | ▴Top |
- Cottini M, Pergolini A, Zampi G, Buffa V, Pino PG, Polizzi V, Ranocchi F, et al. Posterior wall as atypical localization of left atrial myxoma: Diagnosis and management. Herz. 2017;42(4):390-394.
doi pubmed - Pucci A, Gagliardotto P, Zanini C, Pansini S, di Summa M, Mollo F. Histopathologic and clinical characterization of cardiac myxoma: review of 53 cases from a single institution. Am Heart J. 2000;140(1):134-138.
doi pubmed - Kono T, Koide N, Hama Y, Kitahara H, Nakano H, Suzuki J, Isobe M, et al. Expression of vascular endothelial growth factor and angiogenesis in cardiac myxoma: a study of fifteen patients. J Thorac Cardiovasc Surg. 2000;119(1):101-107.
doi pubmed - Sakamoto H, Sakamaki T, Kanda T, Tsuchiya Y, Sato M, Sato H, Oyama Y, et al. Vascular endothelial growth factor is an autocrine growth factor for cardiac myxoma cells. Circ J. 2004;68(5):488-493.
doi pubmed - Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore). 2001;80(3):159-172.
doi pubmed - Gurudevan SV, Shah H, Tolstrup K, Siegel R, Krishnan SC. Septal thrombus in the left atrium: is the left atrial septal pouch the culprit? JACC Cardiovasc Imaging. 2010;3(12):1284-1286.
doi pubmed - Zhang N, Lan C, Du Z, Lin G, Zhong Y, Fei J, Liu K, et al. Using computed tomography coronary angiography to differentiate atypical cardiac myxoma from thrombus. J Comput Assist Tomogr. 2025;49(4):595-603.
doi pubmed - Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6(4):219-228.
doi pubmed - Colin GC, Gerber BL, Amzulescu M, Bogaert J. Cardiac myxoma: a contemporary multimodality imaging review. Int J Cardiovasc Imaging. 2018;34(11):1789-1808.
doi pubmed - Roth JE, Conner WC, Porisch ME, Shry E. Sinoatrial nodal artery to right atrium fistula after myxoma excision. Ann Thorac Surg. 2006;82(3):1106-1107.
doi pubmed - Burns AC, Osula S, Harley A, Rashid A. Left circumflex coronary artery to left atrial fistula in a patient with mitral regurgitation after excision of a left atrial myxoma. Ann Thorac Surg. 2001;72(5):1732-1733.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.