| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 5, October 2025, pages 421-432
Echocardiographic Evaluation of Right Heart and Hemodynamic Changes After Transcatheter Secundum Atrial Septal Defect Closure in Adults: A Single-Center Retrospective Study
Jamilah S. AlRahimia, b, c, d, e, Almas S. AlSolamib, d, Renad S. Alghamdib, d, Ritaj S. AlZahranib, d, Aryam M. Bawazeerb, d, Nawal W. Kutobb, d, Rana A.O. Madib, d, Amjad A. SaemAldaharb, d, Waad A. AlSulamib, d, Alhanouf AlOtaibib, d
aDepartment of Cardiology, King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Jeddah, Saudi Arabia
bKing Abdullah International Medical Research Center, Jeddah, Saudi Arabia
cCollege of Medicine, King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
dCollege of Applied Medical Sciences, King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
eCorresponding Author: Jamilah S. AlRahimi, Department of Cardiology, King AbdulAziz Medical City, Ministry of National Guard Health Affairs, Jeddah 21423, Saudi Arabia
Manuscript submitted July 17, 2025, accepted October 2, 2025, published online October 19, 2025
Short title: Echocardiographic Changes After ASD Closure
doi: https://doi.org/10.14740/cr2116
| Abstract | ▴Top |
Background: Transcatheter closure of secundum atrial septal defects (ASDs) is a widely accepted intervention in adults with left-to-right shunting and right heart volume overload. However, the time course and magnitude of cardiac remodeling and functional improvement after closure remain incompletely defined. This study evaluated serial echocardiographic changes in right heart structure and hemodynamics following ASD closure.
Methods: In this single-center retrospective cohort, adults who underwent transcatheter secundum ASD closure between January 2020 and December 2023 were included. Echocardiography was performed at baseline, immediately post-procedure, at 3 to 6 months, and at 1 year. Parameters included right atrial (RA) area and volume index (RAVI), right ventricular (RV) dimensions, pulmonary valve velocities, RV systolic pressure (RVSP), mean pulmonary artery pressure (mPAP), tricuspid annular plane systolic excursion (TAPSE), and tricuspid regurgitation (TR) severity. Analyses used Wilcoxon signed-rank tests, Chi-square tests, repeated-measures analysis of variance (ANOVA)/multivariate ANOVA (MANOVA), and mixed-effects models.
Results: Eighty patients were included (mean age: 42.3 ± 15.0 years; 72.5% female). Immediately after closure, significant reductions were observed in TR Vmax (-20.8 cm/s, P = 0.0005), pulmonary valve Vmax (-32.1 cm/s, P < 0.0001), Vmean (-18.6 cm/s, P < 0.0001), velocity time integral (VTI) (-6.9 cm, P < 0.0001), RA area (-2.6 cm2, P < 0.0001), RAVI (-7.0 mL/m2, P < 0.0001), RVSP (-7.7 mm Hg, P < 0.0001), QP:QS (-0.8, P ≤ 0.0001), and mPAP (-10.0 mm Hg, P = 0.0007). Improvements were sustained at 3 - 6 months (n = 54) and at 1 year (n = 19).
Conclusions: Transcatheter ASD closure in adults results in early and sustained improvements in RA and ventricular remodeling, pulmonary pressures, and TR severity. These findings underscore the role of echocardiography in longitudinal surveillance and support timely intervention in patients with significant shunting (QP:QS > 1.5). Larger multicenter studies with extended follow-up and correlation to clinical outcomes are warranted.
Keywords: Transcatheter; Atrial septal defect; Right heart; Pulmonary pressure; Congenital heart disease
| Introduction | ▴Top |
Atrial septal defect (ASD) is among the most common congenital heart disease encountered in adults, with the secundum type accounting for approximately 70-75% of cases [1]. Many cases remain undiagnosed until adulthood, when patients may present with exertional dyspnea, atrial arrhythmias, right heart volume overload, or paradoxical embolism [2]. Chronic right-sided volume loading can impair exercise capacity, increase arrhythmia risk, and eventually contribute to right heart failure, underscoring the importance of timely closure.
The advent of transcatheter closure has transformed the management of secundum ASDs, providing a minimally invasive alternative to surgical repair with high procedural success rates and low complication risk [3]. In appropriately selected patients, percutaneous device closure eliminates left-to-right shunting and promotes reverse remodeling of the right atrium and right ventricle, along with improvements in pulmonary pressures and functional tricuspid regurgitation (TR) [4].
Echocardiography is central to the management of ASD, from initial diagnosis and defect sizing to serial post-closure follow-up. Prior studies have shown early improvements in RA and RV dimensions, pulmonary pressures, and TR severity after transcatheter closure [5-9]. However, the trajectory and durability of these changes in adults remain incompletely characterized. In particular, volumetric indices such as the right atrial volume index (RAVI), which may better capture atrial remodeling than simple linear or area-based measures, have been under-reported. Furthermore, most published series provide limited longitudinal follow-up beyond the early post-procedural phase.
This study aimed to evaluate echocardiographic and hemodynamic changes at four timepoints: baseline, immediately post-procedure, 3 to 6 months, and 1 year following transcatheter secundum ASD closure in adults. The primary objective was to characterize right heart remodeling, pulmonary hemodynamic changes, and TR severity over time. By including volumetric parameters and applying robust statistical approaches, this study seeks to provide contemporary evidence on the physiologic benefits and durability of transcatheter intervention in an adult cohort.
| Materials and Methods | ▴Top |
Study design and population
This retrospective cohort study was conducted at a tertiary cardiac center in Saudi Arabia. Adult patients (≥ 18 years) who underwent transcatheter closure of a secundum ASD between January 2020 to December 2023, were eligible. All patients underwent transcatheter closure with the Amplatzer septal occluder under combined echocardiographic and fluoroscopic guidance.
Inclusion criteria were: 1) A confirmed diagnosis of secundum ASD with evidence of left-to-right shunting; and 2) Evidence of right heart volume overload, as determined by transthoracic echocardiography (TTE) and/or transesophageal echocardiography (TEE). Exclusion criteria included complex congenital heart disease, severe pulmonary hypertension (defined as mean pulmonary artery pressure (mPAP) ≥ 50 mm Hg or pulmonary vascular resistance > 8 Wood units), or contraindications to device closure.
Echocardiographic assessment
Echocardiographic studies were performed using a standardized protocol on the Philips iE33 system at four timepoints: baseline (pre-procedure), immediately post-procedure, 3 to 6 months, and 1 year. Parameters were analyzed in accordance with the American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI) Recommendations for Cardiac Chamber Quantification [10] and the ASE Guidelines for Echocardiographic Assessment of the Right Heart [11], and included the following: 1) Right heart: right atrial (RA) and right ventricular (RV) dimensions, RA area, RAVI, RV systolic pressure (RVSP), and mPAP; 2) Valve and shunt: TR severity, pulmonary valve velocities (Vmax, Vmean, velocity time integral (VTI)), and QP:QS ratio; 3) Left heart: left atrial volume index (LAVI), left ventricular ejection fraction (LVEF), and left ventricular end-diastolic volume index (LVEDVI); 4) RV systolic function: tricuspid annular plane systolic excursion (TAPSE, cm) and tissue Doppler-derived RV systolic velocity (RV S, cm/s); 5) ASD diameter was measured by echocardiography. Device size was selected in accordance with manufacturer recommendations, typically 4 - 6 mm larger than the maximal ASD diameter, taking into account surrounding rim adequacy and patient anatomy.
Examinations were performed by experienced sonographers and interpreted by board-certified cardiologists blinded to clinical data. Discrepancies were resolved by consensus.
Guideline-directed medical therapy (GDMT) assessment
GDMT records were reviewed at each timepoint (baseline, immediate post-procedure, 3 - 6 months, and 1 year). Classes included beta-blockers, ACE inhibitors/angiotensin receptor blockers/angiotensin receptor-neprilysin inhibitors (ACEI/ARB/ARNI), diuretics, pulmonary vasodilators, and antiarrhythmics. Other cardio-active agents were recorded when applicable. Medication use was summarized descriptively and included in sensitivity analyses.
Clinical outcomes
Functional status was assessed at each follow-up visit using the New York Heart Association (NYHA) functional classification, based on clinician documentation and patient-reported exercise tolerance. Rhythm status was determined from routine 12-lead electrocardiograms (ECG) and review of medical records for documented arrhythmia events. Formal exercise testing, systematic arrhythmia monitoring, and quality-of-life instruments were not performed
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile ranges, depending on distribution. Paired comparisons across timepoints were performed using the Wilcoxon signed-rank test for non-normally distributed variables, and repeated-measures analysis of variance (ANOVA) for normally distributed variables such as LVEF, LV volumes. Multivariate ANOVA (MANOVA) was used to assess joint changes in RA and RV dimensions. Categorical variables, including TR severity, were analyzed using the Chi-square test simplicity and consistency with prior literature. We acknowledge that paired categorical or ordinal regression methods may provide greater precision but were not feasible given the small number of patients with complete 1-year data and sparse category distributions.
To account for attrition and repeated measures, we additionally fitted mixed-effects models with a random intercept for each subject. Fixed covariates included age, sex, and baseline ASD/device size. Sensitivity analyses further incorporated medication category as a covariate. Adjusted estimates with 95% confidence intervals (CIs) are presented for RVSP, mPAP, TR velocity, RAVI, and RV/LV ratio.
Analyses were conducted on available paired data without imputation, as follow-up echocardiograms were not completed in all patients. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using JMP software (SAS Institute, Cary, NC).
Sample size and ethics
Sample size was calculated to detect a medium effect size (Cohen’s d > 0.5) for changes in RVSP, with 80% power and α = 0.05, requiring at least 64 patients for paired comparisons. The study included 81 patients who underwent ASD closure and met this requirement, although follow-up was incomplete for some patients.
The study was approved by the Institutional Review Board of King Abdullah International Medical Research Center (KAIMRC; approval number: 0000045324). It was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Baseline demographic and procedural characteristics
A total of 80 adult patients underwent transcatheter ASD closure. The mean age was 42.3 ± 15.0 years, with 25.0% younger than 30 years, 32.5% between 30 and 45 years, and 42.5% older than 45 years. The majority of patients were female (72.5%). The mean body surface area (BSA) was 1.72 ± 0.23 m2, with nearly half (46.3%) between 1.6 and 1.8 m2. The mean ASD diameters was 2.4 ± 0.6 cm (range: 0.8 - 3.6 cm). Most defects (79%) were in the moderate range (1.8 - 3.2 cm), while 15% were small (< 1.8 cm) and 6% were large (> 3.2 cm). Device sizes ranged from 10 to 40 mm, with moderate defects typically closed using 24 - 36 mm devices, small defects with 16 - 24 mm devices, and large defects with 36 - 40 mm devices.
Procedural success was achieved in all patients (100%), and there were no major complications (e.g., device embolization, cardiac perforation, or emergency surgery), or clinically significant residual shunt at discharge (Table 1).
 Click to view | Table 1. Baseline Demographic and Procedural Characteristics of Patients Undergoing Transcatheter ASD Closure (N = 80) |
Immediate post-procedure changes
Immediately after closure, significant reductions were observed in pulmonary flow indices: TR Vmax decreased by -20.8 cm/s (95% CI: -30.1 to -11.5, P = 0.0005), pulmonary valve Vmax by -32.1 cm/s, (95% CI: -41.2 to -23.0, P < 0.0001), Vmean by -18.6 cm/s, (95% CI: -26.5 to -10.7, P < 0.0001), and VTI by -6.9 cm, (95% CI: -9.2 to -4.6, P < 0.0001). RA remodeling was also evident, with decreases in length (-0.1 cm, 95% CI: -0.2 to -0.01, P = 0.0183), width (-0.6 cm, 95% CI: -0.8 to -0.4, P < 0.0001), area (-2.6 cm2, 95% CI: -3.2 to -2.0, P < 0.0001), and RA volume index (-7.0 mL/m2, 95% CI: -8.9 to -5.1, P < 0.0001). RVSP (-7.7 mm Hg, 95% CI: -9.8 to -5.6, P < 0.0001) and mPAP (-10.0 mm Hg, 95% CI: -14.2 to -5.8, P = 0.0007) also decreased significantly. TAPSE improved (+0.2 cm, 95% CI: +0.1 to +0.3, P < 0.0001). The QP:QS ratio decreased from 1.8 to approximately 1.0 (-0.8, 95% CI: -1.0 to -0.6, P < 0.0001). LVEF, LAVI, LVEDVI, RV/LV ratio, and RV S’ velocity showed no significant changes (P > 0.05) (Table 2). Repeated-measures ANOVA confirmed overall reductions in RA/RV dimensions (F = 12.34, P < 0.001) and pulmonary pressures (F = 15.67, P < 0.001).
 Click to view | Table 2. Immediate Post-Procedural Echocardiographic and Hemodynamic Changes (N = 80) |
Early follow-up (3 - 6 months)
Follow-up data were available for 54 patients. Significant reductions persisted in TR Vmax (-28.9 cm/s, 95% CI: -40.2 to -17.6, P = 0.0006), pulmonary valve Vmax (-34.2 cm/s, 95% CI: -47.8 to -20.6, P = 0.003), Vmean (-21.4 cm/s, 95% CI: -33.6 to -9.2, P = 0.0035), and VTI (-8.9 cm, 95% CI: -13.5 to -4.3, P = 0.0028). RA dimensions decreased further: width (-0.8 cm, 95% CI: -1.0 to -0.6, P < 0.0001), length (-0.2 cm, 95% CI: -0.3 to -0.1, P = 0.0007), RA area (-3.7 cm2, 95% CI: -4.6 to -2.8, P < 0.0001), and RA volume index (-7.9 mL/m2, 95% CI: -10.1 to -5.7, P < 0.0001). RVSP decreased by -11.8 mm Hg (95% CI: -15.4 to -8.2, P = 0.0008), QP:QS by -0.8 (95% CI: -1.0 to -0.6, P = 0.002) and mPAP by -10.8 mm Hg (95% CI: -15.2 to -6.4, P = 0.005). TAPSE, RV/LV ratio, RV size, EF, and LAVI showed no significant changes (Table 3). MANOVA confirmed reductions in combined RA/RV dimensions (Wilks’ Lambda = 0.82, P = 0.002).
 Click to view | Table 3. Echocardiographic Changes at Early (3 - 6 Months) Follow-Up (N = 54) |
One-year follow-up
At 1 year, echocardiographic data were available for 19 patients. Significant reductions were observed in TR Vmax (-4.2 cm/s, 95% CI: -8.4 to -0.01, P = 0.050), pulmonary valve Vmax (-17.9 cm/s, 95% CI: -28.6 to -7.2, P = 0.006), Vmean (-6.2 cm/s, 95% CI: -10.8 to -1.6, P = 0.008), VTI (-3.6 cm, 95% CI: -6.2 to -1.0, P = 0.007), and RV/LV ratio (-0.1, 95% CI: -0.18 to -0.02, P = 0.016). RA remodeling was stable, with no further reduction in RAVI or RA width. RVSP showed a modest increase compared to early follow-up (+6.5 mm Hg, 95% CI: -0.01 to +13.0, P = 0.050) (Table 4).
 Click to view | Table 4. Echocardiographic Changes Between Early Follow-Up and at 1 year (N = 19) |
Baseline vs. 1-year comparison
Among 19 patients with complete 1-year follow-up, RA remodeling parameters demonstrated significant improvement. RA area decreased by -3.4 cm2 (95% CI: -6.7 to -0.1, P = 0.040) and RAVI by -7.5 mL/m2 (95% CI: -14.5 to -0.5, P = 0.036). These changes were accompanied by reductions in TR Vmax (-33.1 cm/s, 95% CI: -64.2 to -2.0, P = 0.036), pulmonary valve Vmax (-52.1 cm/s, 95% CI: -79.0 to -25.2, P = 0.001), Vmean (-27.6 cm/s, 95% CI: -41.8 to -13.4, P = 0.001), VTI (-12.5 cm, 95% CI: -19.6 to -5.4, P = 0.001), RV/LV ratio (-0.2, 95% CI: -0.34 to -0.06, P = 0.013), mPAP (-10.2 mm Hg, 95% CI: -18.8 to -1.6, P = 0.024), and QP:QS (-0.8, 95% CI: -1.4 to -0.2, P = 0.013). RVSP remained significantly lower than baseline (-5.3 mm Hg, 95% CI: -9.6 to -1.0, P = 0.013) (Table 5). These findings confirm effective shunt elimination and pulmonary hemodynamic normalization, with sustained RA and RV reverse remodeling at 1 year. Sensitivity analyses excluding patients with interval TR worsening or newly identified pulmonary disease yielded similar results.
 Click to view | Table 5. Comparison of Baseline and 1-Year Follow-Up Echocardiographic Parameters (N = 19) |
These results are further illustrated in Figure 1, which highlights the temporal trends in Qp:Qs, TR Vmax, and mPAP, together with other right heart remodeling indices.
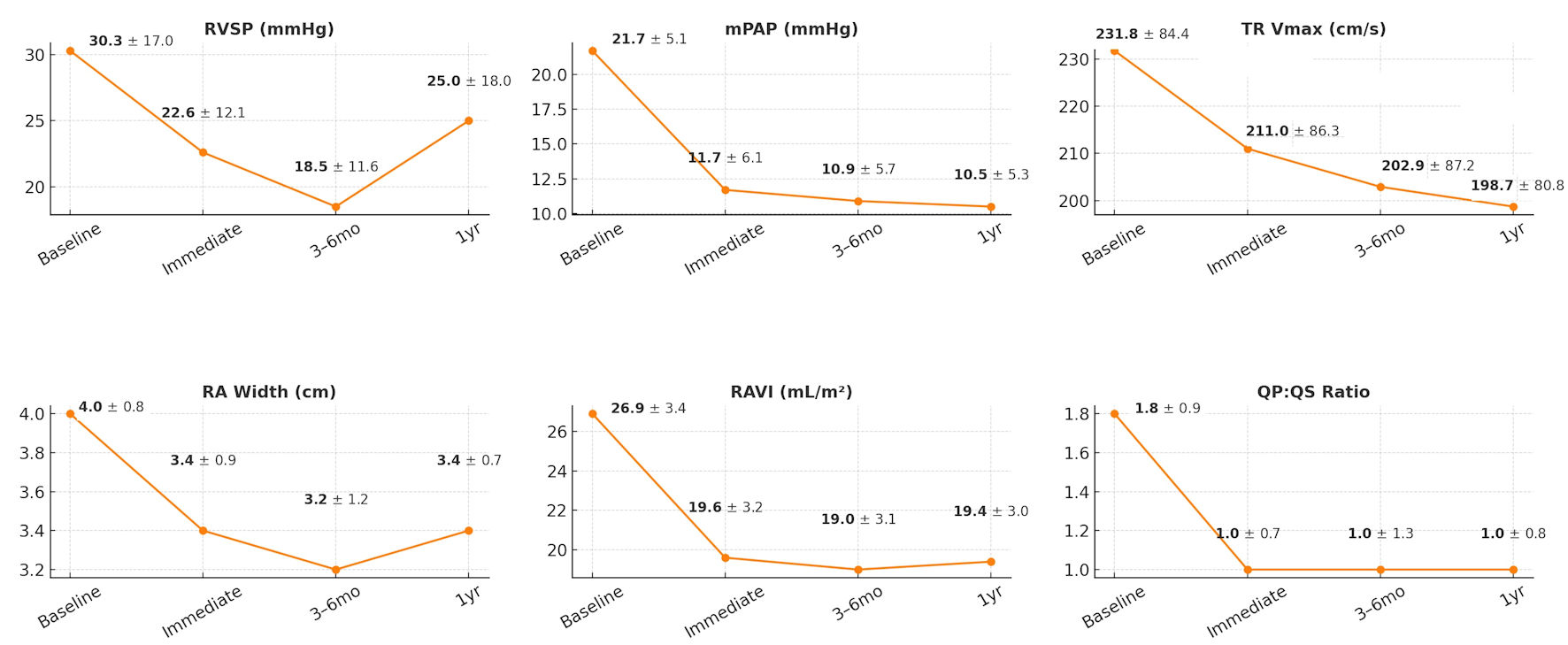 Click for large image | Figure 1. Serial echocardiographic and hemodynamic changes following transcatheter ASD closure. Line graphs demonstrate changes in right ventricular systolic pressure (RVSP), mean pulmonary artery pressure (mPAP), tricuspid regurgitation velocity (TR Vmax), right atrial (RA) width, right atrial volume index (RAVI), and QP:QS ratio from baseline through immediate post-procedure, at 3 - 6 months, and at 1-year follow-up. Values are shown as mean ± standard deviation. Significant reductions were observed in RVSP, mPAP, TR Vmax, RA dimensions, and RAVI after ASD closure, with improvements maintained at follow-up. mo: months; yr: year; ASD: atrial septal defect. |
TR severity
TR severity improved significantly at all follow-up intervals. Compared to baseline, TR grade distributions shifted toward lower severity immediately post-procedure (n = 80, χ2 = 48.82, P < 0.0001), at 3 - 6 months (n = 54, χ2 = 50.89, P < 0.0001), at 1 year (n = 19, χ2 = 35.82, P = 0.003), and in baseline to 1 year paired comparisons (n = 19, χ2 = 26.27, P = 0.010) (Table 6). The distribution of TR severity across timepoints is summarized in Figure 2.
 Click to view | Table 6. Change in Tricuspid Regurgitation Severity Across Timepoints (Chi-square Analyses) |
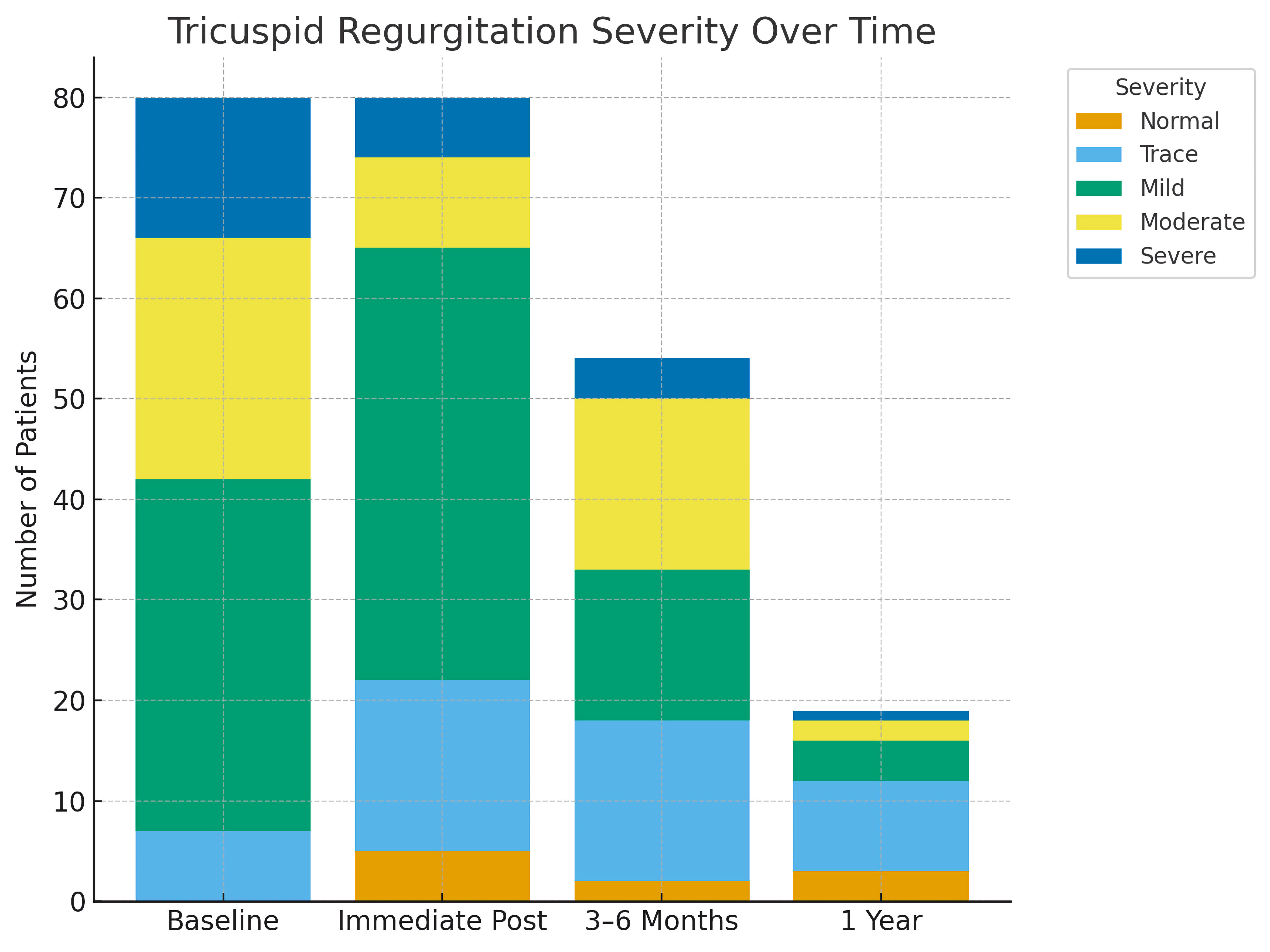 Click for large image | Figure 2. Distribution of tricuspid regurgitation (TR) severity before and after transcatheter ASD closure across follow-up intervals. The stacked bar graph illustrates the proportion of patients with normal, trace, mild, moderate, and severe TR at baseline (pre-procedure), immediately post-procedure, at early follow-up (3 - 6 months), and at 1-year follow-up. A significant reduction in moderate-to-severe TR was observed immediately after the procedure, with sustained improvement during follow-up. ASD: atrial septal defect. |
Mixed-effects models
In adjusted mixed-effects models accounting for age, sex, and baseline ASD/device size, significant improvements were observed across multiple echocardiographic parameters. RVSP and mPAP decreased immediately after closure, with reductions sustained at 3 - 6 months and attenuated but still significant at 1 year. TR velocity and RV/LV ratio followed a similar trajectory, showing consistent improvement across all timepoints. Importantly, RAVI also demonstrated significant reductions, reflecting early and sustained reverse remodeling of the right atrium. Sensitivity analyses incorporating medication category as an additional covariate yielded estimates of similar magnitude, confirming the robustness of these findings (Table 7).
 Click to view | Table 7. Adjusted Mixed-Effects Models for Key Echocardiographic Parameters |
Left heart function
Left ventricular systolic function (EF) and LV volumes (LVEDVI) remained stable across all timepoints (P > 0.05). Similarly, LAVI did not change significantly (P > 0.05) (Tables 2-5).
Cardio-active medications
Medication use remained stable from baseline through follow-up (Table 8). About one-third of patients were on diuretics, and one-quarter on ACEI/ARB/ARNI or beta-blockers. Pulmonary vasodilators and antiarrhythmics were less common (< 15%). Sensitivity analyses adjusting for medication category yielded findings consistent with primary models.
 Click to view | Table 8. Cardio-Active Medication Use Across Follow-Up |
Clinical outcomes
At 3 - 6 months, 98.8% of patients with available follow-up (53/54) showed improvement in NYHA functional class and reported better exercise tolerance. These improvements were maintained at later follow-up. All patients remained in sinus rhythm on available ECGs, with no arrhythmia events documented in the medical records.
| Discussion | ▴Top |
This study evaluated echocardiographic and hemodynamic changes following transcatheter ASD closure in an adult population, with serial follow-up up to 1 year. The results demonstrate significant reduction in pulmonary pressure, improvement in right heart remodeling, and amelioration of TR severity. Procedural success was achieved in all patients without major complications or residual shunts, and at 1 year, sustained reverse remodeling of the right heart confirmed the durable efficacy of the intervention.
Our findings are consistent with prior evidence that percutaneous ASD closure is safe and effective in adults. Improvements in right heart chamber size, RA volume index, and pulmonary hemodynamics were evident immediately after closure and persisted at 1 year, underscoring the physiological benefit of eliminating left-to-right shunting before irreversible right heart dysfunction develops [12, 13].
Reductions in RVSP and mPAP further confirm reversal of right-sided pressure and volume overload. Although a modest rise in RVSP was observed at 1 year, it remained significantly below baseline. This small increase may reflect physiologic variability, changes in loading conditions, or the limited sample size at later follow-up. Overall, these hemodynamic improvements translate into clinical benefits, including improved exercise tolerance, reduced heart failure symptoms, and lower arrhythmia risk [14, 15].
RA and RV volume and dimensions improved significantly, consistent with structural reverse remodeling. Reductions in the RAVI across all follow-up intervals provide robust evidence of unloading and remodeling at the atrial level, complementing decreases in RA area and width. These results underscore the value of RAVI as a sensitive marker for chamber remodeling and support its role in serial surveillance. The transient increase in RV length observed immediately post-procedure may reflect early adaptive remodeling, a finding reported in earlier series [16]. Importantly, RV systolic function, as reflected by TAPSE, improved after closure despite stable RV S’ velocity, indicating enhanced contractile performance due to reduced afterload [17].
TR severity improved consistently, with many patients shifting from mild-moderate to trace or normal. This supports the concept that functional TR in ASD is largely secondary to right-sided dilation and can regress with chamber unloading [18-20]. While TR changes were analyzed with Chi-square methods that do not fully account for paired ordinal data, the consistent trend across all intervals supports the robustness of this finding.
In contrast, LV systolic function and volumes remained stable. This highlights the relative independence of LV performance in this adult cohort, although longer-term studies using LV strain may better characterize subtle inter-ventricular interactions.
Medication use in this cohort remained stable across all timepoints, and sensitivity analyses adjusting for medication category yielded results consistent with the primary models. This suggests that the observed right heart reverse remodeling was unlikely to be driven by changes in pharmacotherapy. In line with guideline-directed medical therapy for heart failure and pulmonary hypertension, patients continued their baseline cardio-active medications without major adjustments, minimizing the risk of treatment-related bias [21]. Nonetheless, residual confounding cannot be fully excluded, and the potential influence of concurrent medical therapy on post-closure remodeling warrants further investigation in larger prospective cohorts.
Most patients reported symptomatic improvement and better NYHA class within 3 - 6 months, which was maintained at 1 year. All remained in sinus rhythm on ECG. The absence of structured arrhythmia monitoring, formal exercise testing, or quality-of-life measures limited correlation of echocardiographic findings with comprehensive clinical outcomes.
Recent studies provide further insight into outcomes across age groups. Meta-analytic data confirm that closure in elderly patients is feasible and beneficial, although the degree of remodeling may be attenuated by comorbidities [22]. Large-scale population studies have demonstrated improved survival and reductions in heart failure and stroke after closure compared with matched controls [23]. These findings support the prognostic benefit of intervention across age groups, while emphasizing that earlier closure may maximize benefit.
Contemporary real-world data also reinforce procedural safety and sustained improvements in right heart function, consistent with the excellent outcomes observed in this study [24]. Together, these complementary reports strengthen the external validity of our findings and confirm reproducibility across diverse healthcare systems.
Nonetheless, diagnosis of ASD in adults is frequently delayed, particularly in patients with comorbidities or ventricular dysfunction. Reports highlight that ASDs may be under-recognized in patients presenting with heart failure or pulmonary hypertension, leading to missed opportunities for timely closure [25]. This underscores the importance of comprehensive echocardiographic evaluation, guided by ASE/EACVI recommendations, to ensure accurate diagnosis, appropriate device selection, and structured follow-up [10, 11].
A key strength of this study is the blinded echocardiographic analysis and the use of robust statistical methods, including mixed-effects models accounting for attrition and baseline covariates. Sensitivity analyses confirmed the stability of the findings.
In summary, transcatheter ASD closure in adults was associated with favorable reverse remodeling, normalization of pulmonary hemodynamics, and excellent procedural safety, with sustained benefit at 1 year. When integrated with recent meta-analyses and population-based studies, these results reinforce that percutaneous closure provides durable clinical and hemodynamic improvements across age groups. Echocardiography remains central for post-procedural surveillance, and in line with international practice guidelines, structured follow-up should include serial imaging at 1 month, 6 - 12 months, and periodically thereafter to monitor remodeling, device position, and valve function [26-28].
Future research should focus on larger multicenter cohorts with longer follow-up, integration of clinical outcomes such as exercise capacity and arrhythmia burden, and advanced statistical methods for categorical outcomes.
Limitations
This study has several limitations. Its single-center, retrospective design and considerable loss to follow-up restrict the generalizability of long-term findings. Only 54 patients had early post-procedural data and 19 had 1-year follow-up, which reduces the precision of durability estimates and introduces potential attrition bias. The small 1-year cohort also limited the use of more robust statistical methods for categorical outcomes; TR severity was analyzed using Chi-square methods, which do not fully account for paired and ordinal data. Although ordinal regression would have been preferable, sparse category distributions precluded its application. Future studies in larger cohorts applying such approaches may yield stronger estimates. The modest increase in RVSP at 1 year should likewise be interpreted with caution given the small sample size. Clinical outcomes were assessed through routine chart review, with functional status determined clinically rather than by standardized exercise testing, and arrhythmia outcomes derived from ECGs without systematic rhythm monitoring or quality-of-life assessments. Finally, the high rate of missed follow-ups was largely related to logistical challenges such as travel distance, scheduling conflicts, and the coronavirus disease 2019 (COVID-19) pandemic period, rather than adverse clinical events. Nonetheless, attrition may have introduced bias if patients with persistent symptoms or complications were underrepresented at later timepoints.
Conclusions
Transcatheter closure of secundum ASDs in adults results in early and sustained improvements in right heart structure, pulmonary pressures, and TR severity, accompanied by symptomatic benefit. These findings highlight the importance of early detection, timely intervention, and structured longitudinal surveillance. Echocardiography remains central for assessing procedural success and long-term remodeling, in line with current ASE/EACVI guideline recommendations, reinforcing its role as the cornerstone of follow-up. In appropriately selected adult patients with significant left-to-right shunting, transcatheter closure provides a safe and effective therapeutic option. Larger multicenter studies with standardized clinical outcome measures, guideline-directed follow-up protocols, and longer observation periods are warranted to confirm and extend these observations.
Acknowledgments
None to declare.
Financial Disclosure
No financial support or funding was received for this study.
Conflict of Interest
The authors declare no conflict of interest related to this work.
Informed Consent
The requirement for informed consent was waived due to the retrospective design of the study.
Author Contributions
JA conceptualized and supervised the study. AA, RA, RZ, AB, NK, and RM contributed to data collection. JA, and WA performed statistical analysis. JA, AS, and AO drafted the manuscript. All authors reviewed and approved the final manuscript and agreed to be accountable for all aspects of the work.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. 2010;31(23):2915-2957.
doi pubmed - Rao PS. Role of echocardiography in the diagnosis and interventional management of atrial septal defects. Diagnostics (Basel). 2022;12(6):1494.
doi pubmed - Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K, Amplatzer I. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39(11):1836-1844.
doi pubmed - Salehian O, Horlick E, Schwerzmann M, Haberer K, McLaughlin P, Siu SC, Webb G, et al. Improvements in cardiac form and function after transcatheter closure of secundum atrial septal defects. J Am Coll Cardiol. 2005;45(4):499-504.
doi pubmed - Kiani R, Firoozbakhsh P, Dokhani N, Alizadehasl A, Bakhshandeh H, Firouzi A, Zahedmehr A, et al. Comparing the impact of transcatheter ASD closure on echocardiographic indices in adults below and above 50 years. Echo Res Pract. 2025;12(1):10.
doi pubmed - Bosshardt D, Voskuil M, Krings GJ, Molenschot MMC, Suttorp MJ, van der Zwaan HB, Post MC. Echocardiographic right ventricular remodeling after percutaneous atrial septal defect closure. Int J Cardiol Congenit Heart Dis. 2023;12:100459.
doi pubmed - Bigdelu L, Nezhad Biglari N, Ghaderi Y, Azari A, Emadzadeh M, Moohebati M, Azadi N, et al. Trans-thoracic echocardiographic findings after the closure of ostium secundum atrial septal defect: A six-month follow-up study. J Cardiovasc Thorac Res. 2025;17(1):27-34.
doi pubmed - Shaban GN, Kassem HK, Setiha MESE, Elsheikh RG, Shaban A, Saed IS. Two-dimensional echocardiography in the evaluation of right ventricular systolic function in patients with atrial septal defect before and after closure. Cardiology and Angiology: An International Journal. 2022;11(4):308-322.
doi - Chen Q, Sun XD, Cao H, Zhang GC, Chen LW, Hu YN. Echocardiographic evaluation of changes in cardiac hemodynamics and loading conditions after transthoracic minimally invasive device closure of atrial septal defect. PLoS One. 2015;10(7):e0128475.
doi pubmed - Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14.
doi pubmed - Mukherjee M, Rudski LG, Addetia K, Afilalo J, D'Alto M, Freed BH, Friend LB, et al. Guidelines for the echocardiographic assessment of the right heart in adults and special considerations in pulmonary hypertension: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2025;38(3):141-186.
doi pubmed - Akseer S, Horlick E, Vishwanath V, Hobbes B, Huszti E, Mak S, Lee DS, et al. Prevalence and outcomes of pulmonary hypertension after percutaneous closure of atrial septal defect: a systematic review and meta-analysis. Eur Respir Rev. 2020;29(158):200099.
doi pubmed - Cool CJ, Kamarullah W, Pranata R, Putra ICS, Khalid AF, Akbar MR, Setiabudiawan B, et al. A meta-analysis of atrial septal defect closure in patients with severe pulmonary hypertension: is there a room for poking holes amid debate? Curr Probl Cardiol. 2024;49(1 Pt C):102121.
doi pubmed - Kilinc B, Eroglu AG, Saltik IL. Early and mid-term follow-up results after transcatheter closure of secundum atrial septal defect. Turk Arch Pediatr. 2022;57(6):661-667.
doi pubmed - Hafez MS, Abdelrahman El-Sayed MI, El Sayed MH. Transcatheter atrial septal defect closure before versus after adulthood. J Saudi Heart Assoc. 2022;34(3):148-152.
doi pubmed - Heaton JN, Okoh AK, Suh S, Ozturk E, Salemi A, Waxman S, Tayal R. Safety and efficacy of the amplatzer septal occluder: a systematic review and meta-analysis. Cardiovasc Revasc Med. 2022;37:52-60.
doi pubmed - Ogura S, Takaya Y, Akagi T, Nakagawa K, Ito H. Percutaneous closure of residual atrial septal defect after surgical closure. Cardiovasc Interv Ther. 2021;36(2):256-259.
doi pubmed - Dreyfus GD, Martin RP, Chan KM, Dulguerov F, Alexandrescu C. Functional tricuspid regurgitation: a need to revise our understanding. J Am Coll Cardiol. 2015;65(21):2331-2336.
doi pubmed - Nassif M, van der Kley F, Abdelghani M, Kalkman DN, de Bruin-Bon R, Bouma BJ, Schalij MJ, et al. Predictors of residual tricuspid regurgitation after percutaneous closure of atrial septal defect. Eur Heart J Cardiovasc Imaging. 2019;20(2):225-232.
doi pubmed - Takaya Y, Akagi T, Kijima Y, Nakagawa K, Ito H. Functional tricuspid regurgitation after transcatheter closure of atrial septal defect in adult patients: long-term follow-up. JACC Cardiovasc Interv. 2017;10(21):2211-2218.
doi pubmed - Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895-e1032.
doi pubmed - Baroutidou A, Arvanitaki A, Farmakis IT, Patsiou V, Giannopoulos A, Efthimiadis G, Ziakas A, et al. Transcatheter closure of atrial septal defect in the elderly: a systematic review and meta-analysis. Heart. 2023;109(23):1741-1750.
doi pubmed - Abrahamyan L, Dharma C, Alnasser S, Fang J, Austin PC, Lee DS, Osten M, et al. Long-term outcomes after atrial septal defect transcatheter closure by age and against population controls. JACC Cardiovasc Interv. 2021;14(5):566-575.
doi pubmed - Thota NR, Kosaraju K, Rudrapogu JS, Nevali KP, Kondaveeti TR. Safety and one-year follow-up analysis of percutaneous ASD closure at a tertiary care hospital. Indian Heart J. 2025;77(3):199-203.
doi pubmed - Sadeghpour A, Kim H, Chamis AL. Undiagnosed atrial septal defect in the setting of comorbidities and ventricular failure: seemingly simple disease with a challenging diagnosis. CASE (Phila). 2023;7(2):72-80.
doi pubmed - Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, Lung B, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42(6):563-645.
doi pubmed - Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(12):1494-1563.
doi pubmed - Plummer ST, Parthiban A, Sachdeva R, Zaidi AN, Statile C. Clinical practice algorithm for the follow-up of unrepaired and repaired secundum atrial septal defects. American College of Cardiology. Published March 8, 2022. Available at: https://www.acc.org/Latest-in-Cardiology/Articles/2022/03/08/19/34/Clinical-Practice-Algorithm-For-the-Follow-Up-of-Unrepaired-and-Repaired-SASD.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.