| Cardiology Research, ISSN 1923-2829 print, 1923-2837 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Cardiol Res and Elmer Press Inc |
| Journal website https://cr.elmerpub.com |
Original Article
Volume 16, Number 5, October 2025, pages 394-402
Evaluation of Gold Marker Orientation in the Three-Cusp Coplanar View After Evolut FX Transcatheter Aortic Valve Implantation
Yusuke Kudoa, Yuta Katoa, d, Yuto Kawahiraa, Midori Miyazakia, b, Tetsuo Hirataa, Hiromitsu Teratanic, Go Kuwaharac, Makoto Sugiharaa, Hideichi Wadac, Masahiro Ogawaa, b, Shin-ichiro Miuraa
aDepartment of Cardiology, Fukuoka University School of Medicine, Jyonan-ku, Fukuoka 814-0180, Japan
bDepartment of Clinical Laboratory and Transfusion, Fukuoka University Hospital, Fukuoka, Japan
cDepartment of Cardiovascular Surgery, Fukuoka University Hospital, Fukuoka, Japan
dCorresponding Author: Yuta Kato, Department of Cardiology, Fukuoka University School of Medicine, Jyonan-ku, Fukuoka 814-0180, Japan
Manuscript submitted July 25, 2025, accepted September 16, 2025, published online October 10, 2025
Short title: Gold Marker Orientation in Coplanar View
doi: https://doi.org/10.14740/cr2124
| Abstract | ▴Top |
Background: Obtaining commissural alignment in transcatheter aortic valve replacement (TAVR) is important for ensuring coronary access and coronary artery filling, reducing the risk of central leaks, and minimizing leaflet stress. The Evolut FX system has the gold markers placed at the neo-commissures and has demonstrated favorable outcomes. We investigated whether evaluating the orientation of the gold markers in a three-cusp coplanar view (3-CV) after Evolut FX implantation was useful for assessing commissural misalignment (CMA).
Methods: Between April 2023 and December 2024, we included 25 patients who underwent transfemoral TAVR using the Evolut FX for symptomatic severe aortic stenosis. All patients underwent multidetector computed tomography (CT) after TAVR. The native-prosthetic gap (NPG) was defined as the distance between the center of the transcatheter heart valve stent frame and the central gold marker in a 3-CV. We evaluated the association between the NPG and CMA, which was derived from the average misalignment deviation on post-TAVR CT.
Results: The median age was 84 years, 36% were male, and 8% had coronary artery disease. The implanting view was the cusp overlap view (COV) in 11 patients, the near-COV in 11 patients, and the left anterior oblique view in three patients. Of the 22 patients implanted using the COV or near-COV, the gold markers were positioned at “2 left-1 right” in 17 patients. The average misalignment deviation was 18.0° (commissural alignment: eight patients, mild CMA: 13 patients, moderate CMA: two patients, and severe CMA: two patients) and the median NPG was 0.11. In cases with commissural alignment and mild CMA, NPG showed a significant positive correlation with the average misalignment deviation (r = 0.68, P < 0.01), whereas in cases with moderate and severe CMA, the relationship was inverse (r = -0.38, P = 0.62). Further, in cases with commissural alignment and mild CMA, a clockwise misalignment occurred when the central marker was positioned closer to the non-coronary cusp side, while a counterclockwise misalignment was observed when positioned closer to the left-coronary cusp side.
Conclusions: Evaluating the orientation of the gold markers in a 3-CV after Evolut FX implantation is useful for assessing CMA.
Keywords: Commissure alignment; Gold marker; Aortic stenosis; Transcatheter aortic valve replacement
| Introduction | ▴Top |
Transcatheter aortic valve replacement (TAVR) is a less invasive alternative to surgical aortic valve replacement (SAVR) [1]. Since recent studies have shown that TAVR is effective in patients of all risk categories, TAVR has become the dominant therapy for patients with severe aortic stenosis (AS) [2-4]. As TAVR indications expand to all-risk and younger patients with longer life expectancy, concerns about the valve durability and coronary access for coronary angiography (CAG) and percutaneous coronary intervention (PCI) after TAVR have been raised [5, 6]. Coronary artery disease (CAD) is often coexistent with AS, the prevalence of which ranges from 30% to 45% in patients with AS undergoing TAVR in Japan [7, 8].
Commissural alignment describes the relationship between transcatheter heart valve (THV) neo-commissures and native aortic valve commissures. The geometric interaction between the neo-commissures and the coronary ostia, including the commissural posts of the THV facing the coronary ostia, makes coronary access challenging [5]. In addition, commissural misalignment (CMA) is associated with central bioprosthetic aortic regurgitation and increased relative aortic valve mean gradient, resulting in structural valve deterioration [9, 10]. Considering these issues after TAVR, avoiding CMA is important for not only ensuring coronary access or coronary artery filling but also THV performance.
The latest Evolut FX system (Medtronic, Dublin, Ireland) has the gold markers placed at the neo-commissures and has demonstrated favorable outcomes, including improved commissural alignment and more symmetrical implantation by adding the hat marker orientation or cusp overlap technique [11]. RE-ACCESS 2 (Reobtain Coronary Ostia Cannulation Beyond Transcatheter Aortic Valve Stent 2) reported that the main cause of unsuccessful coronary cannulations was CMA, with an incidence of approximately 5.5% [12], and the Evolut FX TAVR system achieved more than 95% commissure alignment [11].
A three-cusp coplanar view (3-CV) is determined when the nadirs of the three aortic valve cusps are aligned in the same straight two-dimensional (2D) projected plane on pre-TAVR computed tomography (CT). Aortography in a 3-CV visualizes the orientation of the native aortic valve cusps including each commissure. We investigated whether evaluating the orientation of the gold markers in a 3-CV after Evolut FX implantation was useful for assessing CMA.
| Materials and Methods | ▴Top |
Study population
This study is an observational cohort study of severe AS patients undergoing TAVR at our institution, between April 2023 and December 2024. The Evolut FX system became commercially available for use from April 2023 in Japan. We included symptomatic severe AS patients who underwent transfemoral TAVR using the Evolut FX system and had undergone multidetector CT both before and after TAVR. Exclusion criteria included previous SAVR, alternative access, and bicuspid aortic valve (BAV). This study was performed according to the Declaration of Helsinki regarding investigations in humans and approved by the ethics committee of Fukuoka University Hospital (EC/IRB: U24-11-006).
TAVR procedure
All TAVR procedures were performed via a transfemoral approach, with general anesthesia and transesophageal echocardiographic monitoring. Initially, aortography was performed in the 3-CV derived from pre-TAVR CT. The 3-CV and cusp overlap view (COV) were identified on 3mensio (Pie Medical Imaging, Maastricht, the Netherlands). The COV is defined on pre-TAVR CT as the projection in which the left coronary cusp (LCC) and right coronary cusp (RCC) are overlapped, thereby isolating the non-coronary cusp (NCC). The Evolut FX delivery system was introduced through the femoral artery, positioning the flush port at the 3 o’clock orientation, and advanced to the descending aorta. The orientation of the hat marker was confirmed using fluoroscopy in a left anterior oblique (LAO) view, and it should ideally be positioned on the outer curve (OC). If the hat marker was not located on the OC, a gentle counter-clockwise rotation of the delivery catheter was applied to adjust the hat marker to the OC. Before releasing the THV at annulus, we confirmed that the hat marker was positioned at the center front (CF) in the COV. If extreme angulations of more than 30° right anterior oblique (RAO) and/or 30° caudal were required for the COV, the near-COV with less extreme angulation was used. To eliminate inflow parallax, implant depth at the NCC was verified in the COV, while implant depth at the LCC was verified in the LAO view. We confirmed whether the orientation of the gold markers in the COV was “2 left-1 right” or not; however, if the orientation was not 2 left-1 right, we did not attempt to reorient them. Finally, the THV orientation images were obtained at the 3-CV on fluoroscopy after the THV deployment. We confirmed the orientation of both the C-tab (inner curve (IC), CF, OC, and center back (CB)) and the gold markers [6, 13].
Fluoroscopy and CT analysis after TAVR
On fluoroscopy, positions of the THV neo-commissures were identified using the orientation of the gold markers in the same coplanar view as the initial aortography. First, in a 3-CV, the distance from the edge of the THV stent frame inflow to the central gold marker was measured and defined as “x”. Next, the distance from edge to edge of the THV stent frame inflow was measured and defined as “y”. If the central gold marker was not located on the line extending between the edges of the THV stent frame, its position was determined by projecting it perpendicularly onto this line. The center of the stent frame lies at a distance of y/2 from the end of the THV stent frame. The native-prosthetic gap (NPG) was calculated as |x-y/2|/y, representing the proportion of the distance between the center of the THV stent frame and the central gold marker relative to the total length of the THV stent frame (Fig. 1).
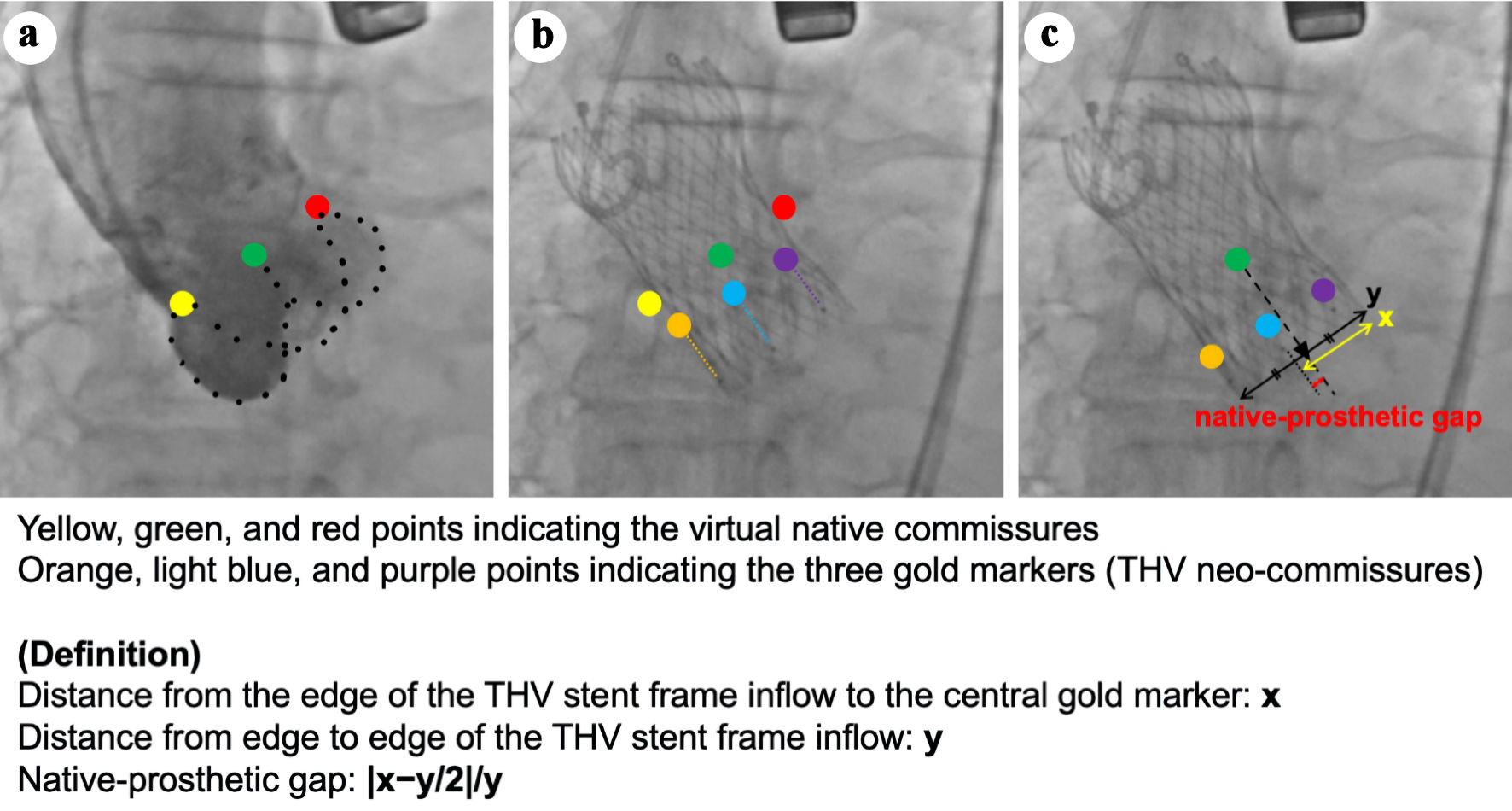 Click for large image | Figure 1. Native-prosthetic gap (NPG). The method for calculating the NPG is as follows. (a) Initial aortography in the three-cusp coplanar view (3-CV) is shown, with yellow, green, and red points indicating the virtual native commissures. (b) The orientation of the gold markers in the 3-CV after transcatheter heart valve (THV) deployment. Orange, light blue, and purple points represent the three gold markers (i.e., THV neo-commissures). (c) The measurement of NPG. The yellow arrow indicates the distance between the end of the THV stent frame and the central gold marker (x), while the black arrow indicates the total length of the THV stent frame (y). The black triangle represents the center of the stent frame, with a distance of y/2 from the end of the THV stent frame. The NPG was calculated as |x-y/2|/y, representing the proportion of the distance between the center of the THV stent frame and the central gold marker relative to the total length of the THV stent frame. 3-CV: three-cusp coplanar view. |
The commissural orientation was evaluated for each patient in the native aortic valve on pre-TAVR CT and in the prosthetic aortic valve on post-TAVR CT during the end-diastolic phase of the cardiac cycle. Commissural alignment was analyzed as previously described [9]. Briefly, first, the right coronary artery (RCA) ostium was identified in a cross-sectional view perpendicular to the axis of the annulus. Next, three angles were measured: from the RCA to the RCC/LCC commissure, from the RCA to the LCC/NCC commissure, and from the RCA to the NCC/RCC commissure. A Δ angle deviation between the pre-TAVR and post-TAVR CT was measured for each of the three angles. The average misalignment deviation was defined as the mean of the absolute values of these three Δ angle deviations, quantifying the overall extent of commissural shift after TAVR. Commissural alignment was categorized into four groups based on the differences in commissural orientation between the native and neo-commissures: commissural alignment (0° to 15.0°), mild CMA (15.1° to 30.0°), moderate CMA (30.1° to 45.0°), and severe CMA (45.1° to 60.0°). We evaluated the association between NPG and CMA.
Statistical analysis
Continuous data are reported as median (interquartile range) and categorical data are presented as percentages. To determine the association between NPG and CMA, we conducted both a linear regression analysis with CMA as a continuous variable and a logistic regression analysis with CMA as a categorical variable. At a logistic regression, receiver operating characteristic (ROC) analysis was used to determine the cut-off value for predicting CMA. A two tailed P < 0.05 was considered statistically significant. NPG measurement was conducted by two experienced physicians who were blinded to the patient characteristics. Inter-observer reliability was assessed using concordance correlation coefficient (CCC) and Bland-Altman analysis. All statistical analyses were performed with JMP 16 (SAS Institute, Inc., Cary, NC, USA) and EZR ver. 1.68 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
| Results | ▴Top |
Patient characteristics
During the study period, a total of 68 TAVR procedures were performed, of which 31 patients underwent TAVR using Evolut FX system. After excluding six patients who met the exclusion criteria, 25 patients were included in this study. Table 1 summarizes the clinical characteristics of this study. The median age was 84 years, 36% were male, 44% were New York Heart Association functional classification III or IV, the median Society of Thoracic Surgeons score was 5.3, and 8% had CAD (including both treated and untreated). Pre-TAVR CT showed that the median annulus area was 386 mm2 and the perimeter was 72 mm. On postprocedural transthoracic echocardiography (TTE), left ventricular ejection fraction was 68 %, peak aortic velocity was 1.7 m/s, mean aortic pressure gradient was 6 mm Hg, and effective orifice area was 1.7 cm2. Additionally, postprocedural aortic regurgitation was none/trivial in 48%, mild in 44%, and moderate in 8%, all of which were identified as perivalvular leaks. Postprocedural TTE and TAVR CT were performed on the same day, with a median duration of 6 days from TAVR procedure to TTE/CT.
 Click to view | Table 1. Baseline Clinical Characteristics |
TAVR procedure and CT analysis
Implanted THV sizes were as follows: 23 mm in 20%, 26 mm in 36%, 29 mm in 40%, and 34 mm in 4%, respectively (Table 2). The implanting view was the COV in 11 cases (44%), the near-COV in 11 cases (44%), and the LAO view in three cases (12%). Of the 22 cases implanted using the COV or near-COV, the gold markers were positioned at 2 left-1 right in 17 cases. The final C-tab positions on the 3-CV were IC in 20 cases (80%), CF in one case (4%), OC in four cases (16%), and CB in none (0%). The median NPG in all patients was 0.11. Inter-observer reliability in measuring NPG was good as indicated by CCC (95% confidence interval) and limits of agreement from Bland-Altman analyses (Supplemental Material 1, cr.elmerpub.com). On post-TAVR CT, the average misalignment deviation was 18.0° (Table 3). Commissural alignment was achieved in eight cases (32%), mild CMA was observed in 13 cases (52%), moderate CMA was observed in two cases (8%), and severe CMA was present in two cases (8%).
 Click to view | Table 2. Procedure and Fluoroscopic Analysis |
 Click to view | Table 3. CT Analysis |
Among the 17 cases in which the gold markers were positioned at 2 left-1 right in the COV or near-COV, the final C-tab positions on the 3-CV were IC in 16 cases (94%), OC in one case (6%), and CF/CB in none (0%). Median NPG was 0.11, with an average misalignment deviation of 16.0°. Commissural alignment was observed in eight cases (47%), mild CMA in eight cases (47%), moderate CMA in one case (6%), and no cases of severe CMA (Supplemental Material 2, cr.elmerpub.com).
Association between C-tab and NPG with CMA
The association between the final C-tab positions and CMA was as follows: IC, all cases with alignment or mild CMA; CF, severe CMA; and OC, one case with alignment or mild CMA, two cases with moderate CMA, and one case with severe CMA. Among the 17 cases in which the gold markers were positioned at 2 left-1 right in the COV or near-COV, all cases with the C-tab located at IC showed alignment or mild CMA, whereas the single case with the C-tab located at OC showed moderate CMA.
Linear regression analysis for CMA demonstrated that NPG was not correlated with the average misalignment deviation in all cases (r = 0.37, P = 0.07) (Fig. 2a). However, in cases with commissural alignment and mild CMA, NPG showed a significant positive correlation with the average misalignment deviation (r = 0.68, P < 0.01), whereas in cases with moderate and severe CMA, the relationship was inverse (r = -0.38, P = 0.62) (Fig. 2b, c). Figure 2d shows the predictive abilities for CMA as assessed by logistic regression analysis and ROC analysis in cases with commissural alignment and mild CMA. NPG demonstrated high predictive value for CMA, with an area under the curve (AUC) of 0.83 (P < 0.01). The optimal cut-off value for NPG was 0.17, with a sensitivity of 0.62 and a specificity of 1.0. Among the 17 cases in which the gold markers were positioned at 2-left 1-right in the COV or near-COV, NPG showed a strong correlation with the average misalignment deviation (r = 0.86, P < 0.01) (Supplemental Material 3A, cr.elmerpub.com). Furthermore, NPG demonstrated an even higher predictive value for CMA, with an AUC of 0.97 (P < 0.01), and the optimal cut-off value for NPG remained 0.17, with a sensitivity of 0.78 and a specificity of 1.0 (Supplemental Material 3B, cr.elmerpub.com).
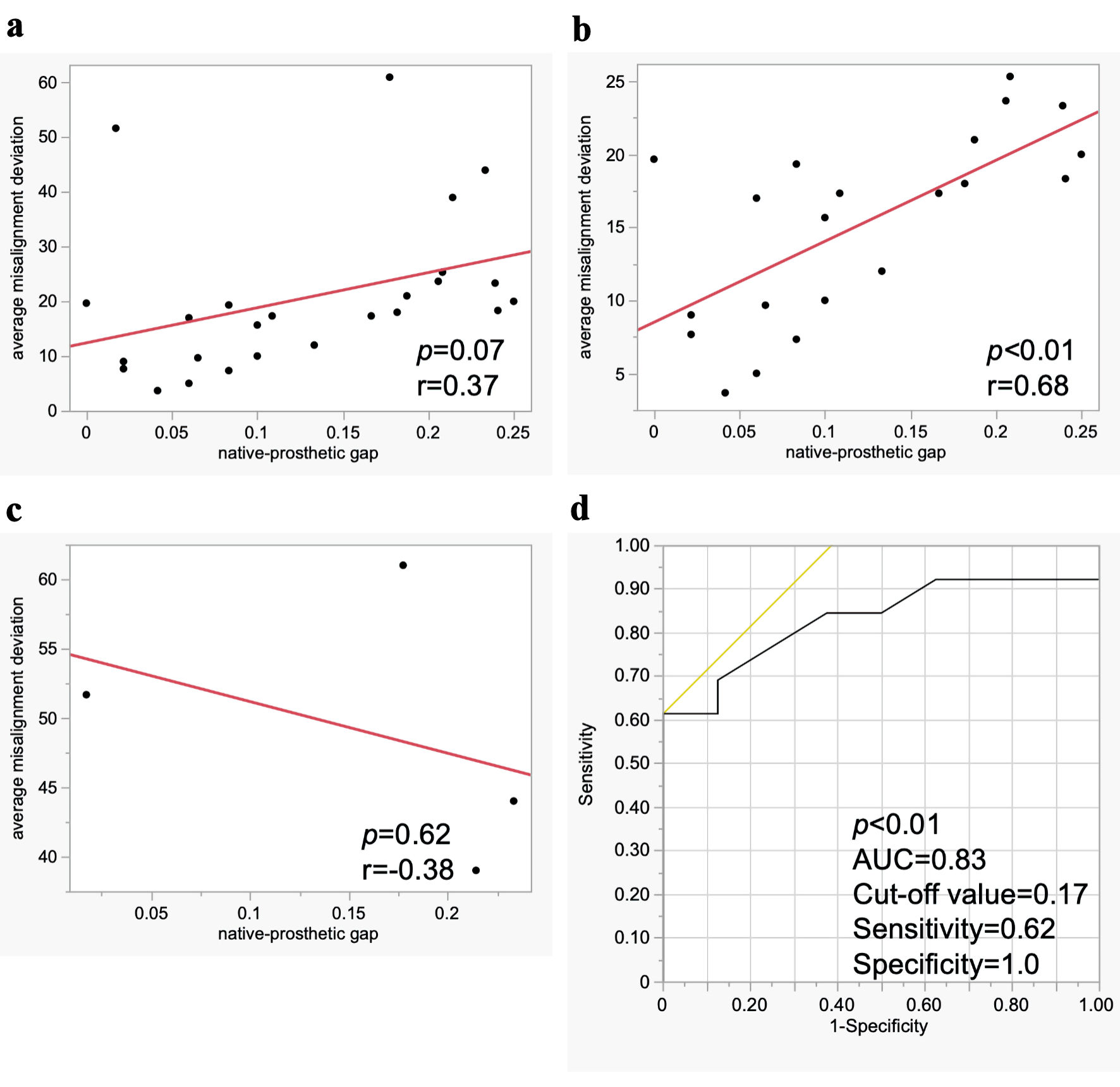 Click for large image | Figure 2. Association between native-prosthetic gap and commissural misalignment (CMA). Linear regression analysis for CMA in all cases (a), in cases with commissural alignment and mild CMA (b), and in cases with moderate and severe CMA (c). Logistic regression and receiver operating characteristic analysis for CMA in cases with commissural alignment and mild CMA (d). AUC: area under the curve. |
Additionally, among patients with commissural alignment and mild CMA, a clockwise misalignment occurred when the central marker was positioned closer to the NCC side. Conversely, a counterclockwise misalignment was observed when the central marker was positioned closer to the LCC side (Fig. 3). In the case of moderate or severe CMA, this finding was not observed.
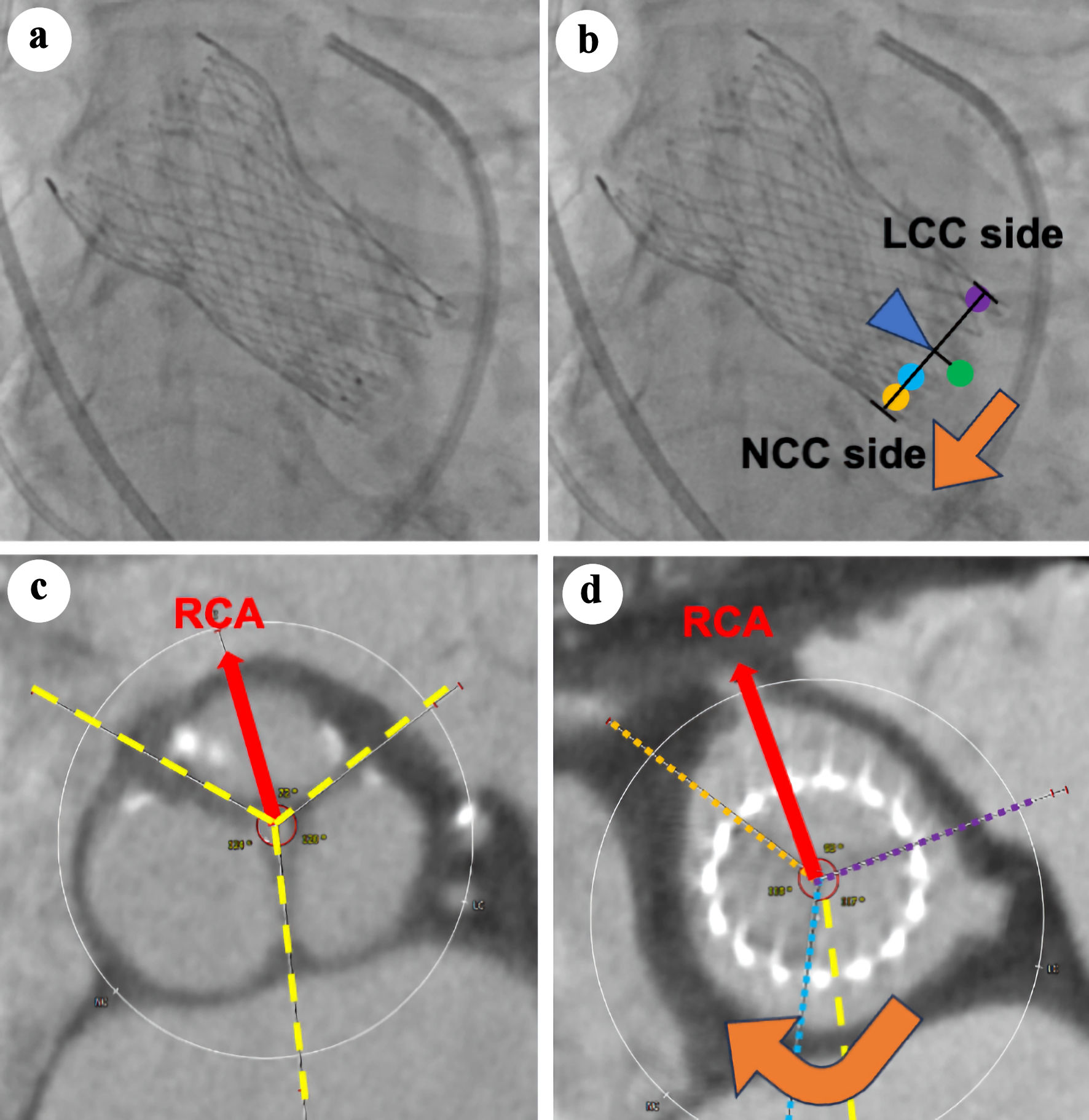 Click for large image | Figure 3. Association between the direction of central marker shift in fluoroscopy and the direction of commissural misalignment in computed tomography (CT). (a, b) The transcatheter heart valve (THV) orientation images obtained in the three-cusp coplanar view on fluoroscopy after THV deployment. Orange, light blue, and purple points represent the three gold markers (i.e., THV neo-commissures). The green point represents the virtual native commissure, located at the center of the THV stent frame. (c, d) Preprocedural and postprocedural CT. The red arrow indicates the direction of the RCA. Yellow dashed lines mark the native commissures, while orange, light blue, and purple dotted lines denote the neo-commissures of the THV. Clockwise misalignment occurred when the central marker was positioned closer to the NCC side (orange arrow, (b, d)). NCC: non-coronary cusp; LCC: left coronary cusp; RCA: right coronary artery. |
| Discussion | ▴Top |
This is a preliminary report to investigate the usefulness of the orientation of the gold marker in a 3-CV after Evolut FX implantation for assessing CMA. The main findings from our study are as follows: 1) The current method for delivery and deployment of the Evolut FX system achieved improved commissural alignment; 2) Fluoroscopy-derived NPG was strongly correlated with CMA in cases with commissural alignment and mild CMA. However, unlike the existing methods, our approach is not intended to improve CMA but rather to assess the degree of CMA under fluoroscopy following valve deployment. Our method is an alternative to post-TAVR CT for evaluating CMA, particularly in patients with advanced chronic kidney disease who should avoid contrast exposure.
ALIGN TAVR has validated a fluoroscopic-CT coregistration technique using pre-TAVR CT and intraprocedural fluoroscopy to determine commissural alignment [13]. They described that positioning the hat marker orientation at the OC or the CF of the annulus in a COV improved commissural alignment and resulted in a lower incidence of coronary artery overlap, compared to positioning the hat marker orientation at the IC or the CB. Positioning the hat marker orientation at the OC or the CF of the annulus in a COV resulted in the C-tab being located at the IC of the aortic root in a 3-CV, leading to better commissural alignment. In the present study as well, all cases in which the C-tab was positioned at the IC obtained favorable commissural alignment. Further, they suggested the usefulness of assessing CMA using two commissural tabs on the COV. However, the accuracy of their technique depends on whether the initial 3-CV is truly coplanar, and it cannot be applied in cases requiring extreme angulations for the COV. In contrast, our method is able to assess both the degree and direction (clockwise or counterclockwise) of CMA using the spatial orientation of the three gold markers. However, our method also has major limitations. First, compared to their approach, our method does not improve CMA, but only allows assessment after TAVR. Second, NPG was significantly correlated with CMA in cases with commissural alignment and mild CMA; however, this correlation was not observed in all cases, particularly in those with moderate and severe CMA. In cases with moderate and severe CMA, the relationship between NPG and CMA was inverse. As the degree of CMA progresses to mild, the central marker tends to move away from the center of the THV stent frame; however, when it reaches moderate and severe, the central marker paradoxically shifts closer to the center of the THV stent frame (Fig. 4). The correlation between NPG and CMA is considered positive as CMA progresses from alignment to mild; however, it becomes inversely correlated as CMA advances to moderate and severe. Therefore, our method alone was limited to mild CMA. When the C-tab was located at the IC, commissural alignment was favorable and positively correlated with NPG, indicating the usefulness of NPG through the assessment of C-tab position. In contrast, when the C-tab was located at positions other than the IC, the severity of CMA could not be determined, and the association between NPG and CMA remained unclear. In cases with moderate and severe CMA, the orientation of the gold markers in the COV was not 2 left-1 right. We propose that the utility of our method will be enhanced by incorporating the assessment of the orientation of the C-tab in the 3-CV and the gold markers in the COV. In another study, an intraprocedural method to evaluate CMA using the en-face (deep RAO cranial) view was reported [14]. This approach can improve CMA before valve deployment; however, obtaining a true en-face view is technically challenging and often requires aortography to evaluate native commissure orientation and coronary ostia.
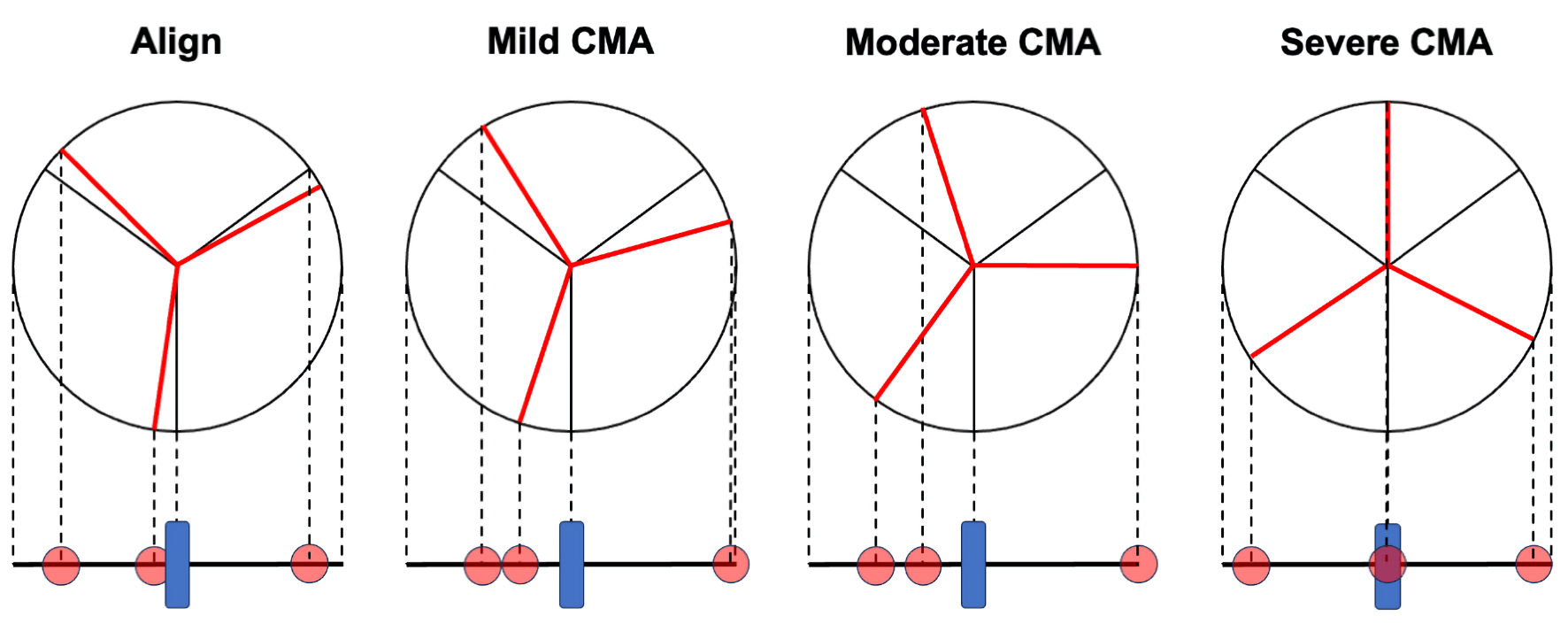 Click for large image | Figure 4. Association between the orientation of the three gold markers and commissural misalignment (CMA). The relationship between CMA, as assessed by computed tomography, and the orientation of the gold markers (red circles) in fluoroscopy. As the degree of CMA progresses to mild, the central marker tends to move away from the center of the transcatheter heart valve (THV) stent frame (blue square). However, as it progresses to moderate and severe, the central marker paradoxically shifts closer to the center of the THV stent frame. |
Previous studies have reported that CMA is associated with central bioprosthetic aortic regurgitation and increased relative aortic valve mean gradient [9, 10]. In this study, most cases were aligned or mild CMA. Therefore, while we cannot assess the impact of CMA on THV performance, Evolut FX demonstrated the favorable outcomes. Incidentally, two cases of postprocedural moderate aortic regurgitation were observed in cases with commissural alignment and mild CMA. As TAVR indications expand to younger patients with longer life expectancy, concerns about THV performance or durability have led to the consideration of redo-TAVR. While coronary alignment and commissural alignment are important for redo-TAVR [6, 13], visualizing the orientation of the neo-commissures with the gold markers may facilitate the BASILICA procedure during redo-TAVR. In these patients, ensuring THV durability and coronary access through commissural alignment is a priority for redo-TAVR and lifetime management.
Limitation
This study has several limitations that warrant discussion. First, our study is a retrospective analysis performed at a single tertiary referral center with a limited number of study patients. The small sample size limits both the statistical power and the generalizability of the findings. Our study did not include patients with BAV or those who have not undergone postprocedural CT due to renal failure among those implanted with the Evolut FX, which may result in selection bias. Second, our method is limited to evaluating misalignment up to mild severity. Our results suggest that moderate or severe misalignment may have an inverse relationship with NPG; however, due to the limited number of those cases, this relationship could not be adequately assessed. Third, a 3-CV indicates that the nadirs of the three aortic valve cusps are aligned in the same straight 2D projected plane on pre-TAVR CT. However, this does not necessarily imply that the native commissures are also aligned in the same plane during TAVR procedure. If discrepancies between the 3-CV derived on pre-TAVR CT and the actual angiographic perpendicular view during TAVR were present, we did not attempt to reorient them, which may have influenced the results of this study. Fourth, the NPG (including x and y) may vary depending on expansion completeness (i.e., circular or elliptical THV) or implantation coaxiality. These potential geometric distortions could have influenced the accuracy of our measurements. Finally, our method relies on assessment after THV deployment without improving CMA. A novel technique or approach that enables evaluation before the final release of the THV would be beneficial for improving CMA and enhancing clinical outcomes in the future.
Conclusions
The latest Evolut FX system has the gold markers placed at the neo-commissures and has shown promising outcomes. In TAVR using Evolut FX system, our method appears to be convenient and less invasive compared to CT and may be useful for the evaluation of CMA. While our method is limited to evaluating CMA only up to mild severity, a new method that can assess all severities before the final THV deployment is expected to be needed in the future.
| Supplementary Material | ▴Top |
Suppl 1. Inter-observer reliability in measuring native-prosthetic gap.
Suppl 2. Procedure and fluoroscopic analysis on 2 left-1 right in cusp overlap or near-cusp overlap view.
Suppl 3. Association between native-prosthetic gap and commissural misalignment in cases where the gold markers were positioned as 2 left-1 right in the cusp overlap or near-cusp overlap views.
Acknowledgments
We thank the members of Heart Team at the Fukuoka University Hospital.
Financial Disclosure
None to declare.
Conflict of Interest
There is no conflict of interest to declare.
Informed Consent
Informed consent was obtained in the form of opt-out on the website.
Author Contributions
Conception and design: Y. Kudo and Y. Kato. Acquisition and analysis of data: Y. Kudo, Y. Kawahira and Y. Kato. Manuscript drafting: Y. Kudo and Y. Kato. Interpretation of data, revising the work critically for important intellectual content: Y. Kato, M. Sugihara, H. Wada, M. Ogawa, and S. Miura. Final approval of the version to be published: all authors. Accountability for all aspects of the work: all authors.
Data Availability
The data presented in this study are available upon request from the corresponding author.
Abbreviations
TAVR: transcatheter aortic valve replacement; SAVR: surgical aortic valve replacement; AS: aortic stenosis; CAG: coronary angiography; PCI: percutaneous coronary intervention; CAD: coronary artery disease; THV: transcatheter heart valve; CMA: commissural misalignment; 3-CV: three-cusp coplanar view; 2D: two-dimensional; CT: computed tomography; BAV: bicuspid aortic valve; COV: cusp overlap view; LCC: left coronary cusp; RCC: right coronary cusp; NCC: non-coronary cusp; LAO: left anterior oblique; RAO: right anterior oblique; NPG: native-prosthetic gap; RCA: right coronary artery; ROC: receiver operating characteristic; CCC: concordance correlation coefficient; TTE: transthoracic echocardiography; AUC: area under the curve
| References | ▴Top |
- Spears J, Al-Saiegh Y, Goldberg D, Manthey S, Goldberg S. TAVR: a review of current practices and considerations in low-risk patients. J Interv Cardiol. 2020;2020:2582938.
doi pubmed - Khan SU, Riaz H, Khan MU, Zarak MS, Khan MZ, Khan MS, Sattur S, et al. Meta-analysis of temporal and surgical risk dependent associations with outcomes after transcatheter versus surgical aortic valve implantation. Am J Cardiol. 2019;124(10):1608-1614.
doi pubmed - Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, Bajwa T, et al. 3-year outcomes after transcatheter or surgical aortic valve replacement in low-risk patients with aortic stenosis. J Am Coll Cardiol. 2023;81(17):1663-1674.
doi pubmed - Mack MJ, Leon MB, Thourani VH, Pibarot P, Hahn RT, Genereux P, Kodali SK, et al. Transcatheter aortic-valve replacement in low-risk patients at five years. N Engl J Med. 2023;389(21):1949-1960.
doi pubmed - Ochiai T, Chakravarty T, Yoon SH, Kaewkes D, Flint N, Patel V, Mahani S, et al. Coronary access after TAVR. JACC Cardiovasc Interv. 2020;13(6):693-705.
doi pubmed - Tang GHL, Amat-Santos IJ, De Backer O, Avvedimento M, Redondo A, Barbanti M, Costa G, et al. Rationale, Definitions, Techniques, and Outcomes of Commissural Alignment in TAVR: From the ALIGN-TAVR Consortium. JACC Cardiovasc Interv. 2022;15(15):1497-1518.
doi pubmed - Yamamoto M, Watanabe Y, Tada N, Naganuma T, Araki M, Yamanaka F, Mizutani K, et al. Transcatheter aortic valve replacement outcomes in Japan: Optimized CathEter vAlvular iNtervention (OCEAN) Japanese multicenter registry. Cardiovasc Revasc Med. 2019;20(10):843-851.
doi pubmed - Kamon T, Kaneko H, Kiriyama H, Itoh H, Fujiu K, Kumazawa R, Morita K, et al. Transcatheter aortic valve implantation and surgical aortic valve replacement for aortic stenosis in Japan - analysis of a nationwide inpatient database. Circ Rep. 2020;2(12):753-758.
doi pubmed - Fuchs A, Kofoed KF, Yoon SH, Schaffner Y, Bieliauskas G, Thyregod HG, Makkar R, et al. Commissural alignment of bioprosthetic aortic valve and native aortic valve following surgical and transcatheter aortic valve replacement and its impact on valvular function and coronary filling. JACC Cardiovasc Interv. 2018;11(17):1733-1743.
doi pubmed - Raschpichler M, Flint N, Yoon SH, Kaewkes D, Patel C, Singh C, Patel V, et al. Commissural Alignment After Balloon-Expandable Transcatheter Aortic Valve Replacement Is Associated With Improved Hemodynamic Outcomes. JACC Cardiovasc Interv. 2022;15(11):1126-1136.
doi pubmed - Zaid S, Attizzani GF, Krishnamoorthy P, Yoon SH, Palma Dallan LA, Chetcuti S, Fukuhara S, et al. First-in-human multicenter experience of the newest generation supra-annular self-expanding evolut FX TAVR system. JACC Cardiovasc Interv. 2023;16(13):1626-1635.
doi pubmed - Costa G, Sammartino S, Strazzieri O, Motta S, Frittitta V, Dipietro E, Comis A, et al. Coronary Cannulation Following TAVR Using Self-Expanding Devices With Commissural Alignment: The RE-ACCESS 2 Study. JACC Cardiovasc Interv. 2024;17(6):727-737.
doi pubmed - Tang GHL, Zaid S, Fuchs A, Yamabe T, Yazdchi F, Gupta E, Ahmad H, et al. Alignment of Transcatheter Aortic-Valve Neo-Commissures (ALIGN TAVR): impact on final valve orientation and coronary artery overlap. JACC Cardiovasc Interv. 2020;13(9):1030-1042.
doi pubmed - Hirose S, Nakashima M, Enta Y, Ishii K, Toyoda S, Tada N. Intraprocedural assessment of commissural alignment of the Navitor system using the en-face view. Cardiovasc Interv Ther. 2023;38(3):356-357.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cardiology Research is published by Elmer Press Inc.