A Clinical Retrospective Study on the Combined Use of Monocyte-to-Lymphocyte Ratio and Triglyceride-Glucose Index to Predict the Severity of Coronary Artery Disease
DOI:
https://doi.org/10.14740/cr2006Keywords:
Monocyte-to-lymphocyte ratio, Triglyceride-glucose index, Coronary artery disease, Risk prediction, AtherosclerosisAbstract
Background: Coronary artery disease (CAD) remains a leading cause of morbidity and mortality. Traditional risk models based on factors like age, hypertension, and lipid levels are limited in individualized prediction, especially for high-risk populations. This study evaluates the independent and combined predictive value of the monocyte-to-lymphocyte ratio (MLR) and triglyceride-glucose (TyG) index for assessing CAD severity.
Methods: In this single-center, retrospective study, 678 patients who underwent coronary angiography (CAG) between January 2022 and June 2024 were included. Eligible patients were aged ≥ 40 years with suspected or confirmed CAD. Clinical data and laboratory values were extracted from electronic records. MLR was calculated as the monocyte-to-lymphocyte ratio, and TyG index was derived from fasting triglycerides and glucose. CAD severity was categorized by SYNTAX scores into no CAD, mild, moderate, and severe CAD. Statistical analyses included Spearman correlation, multivariate logistic regression, and receiver operating characteristic (ROC) curve analysis to assess the diagnostic accuracy of MLR and TyG index.
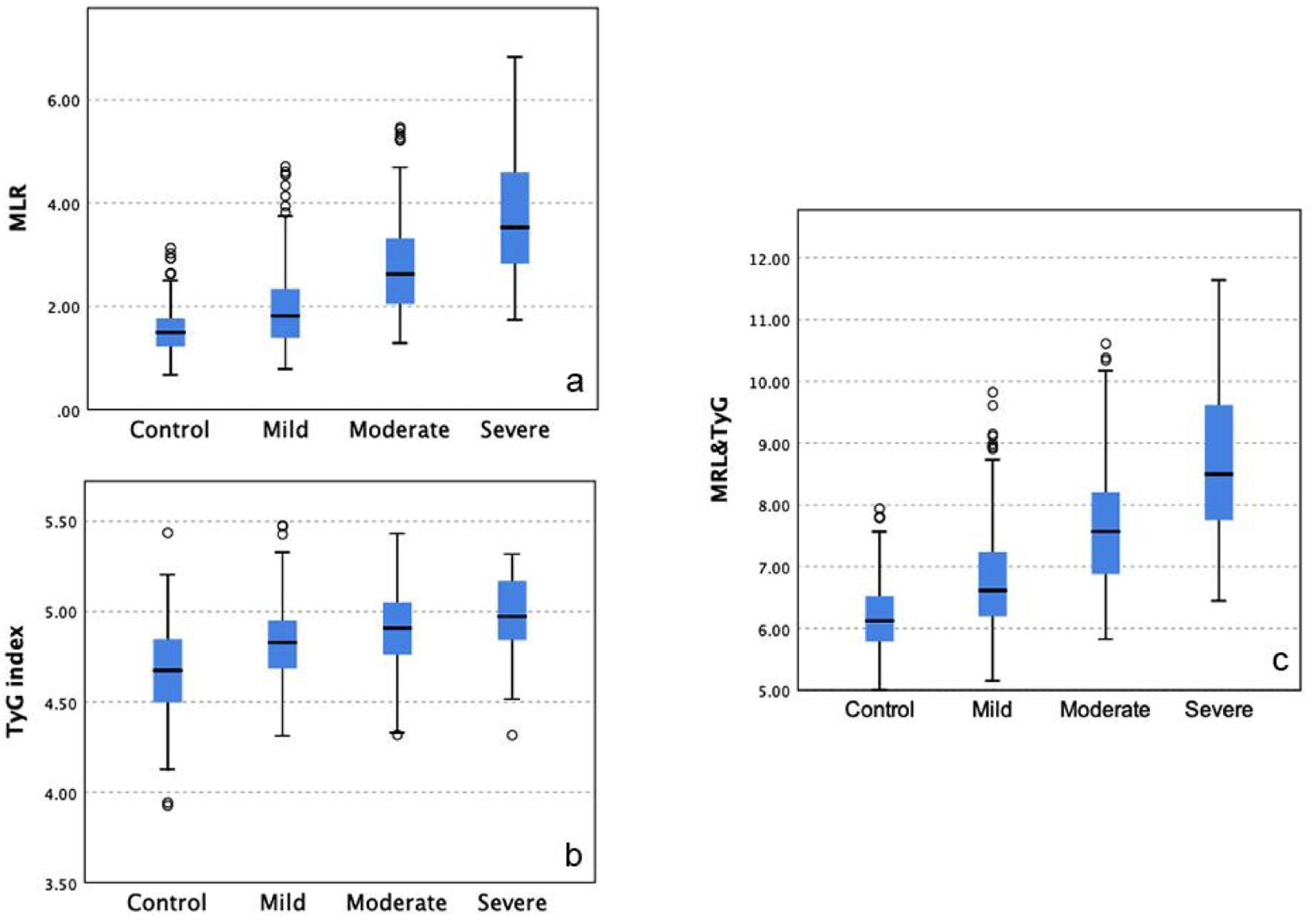
Results: Of the 678 patients, 67.1% had CAD. Both MLR and TyG index were significantly associated with CAD severity, with MLR showing a stronger correlation with SYNTAX scores. Multivariate analysis confirmed MLR (odds ratio (OR) = 2.15) and TyG index (OR = 1.75) as independent predictors of CAD. The combined MLR-TyG model achieved an area under the curve (AUC) of 0.804, surpassing the predictive value of each marker alone. Subgroup analysis indicated high predictive accuracy in diabetic and hypertensive patients.
Conclusions: MLR and TyG index independently and jointly predict CAD severity, with the combined model enhancing diagnostic accuracy. Reflecting both inflammatory and metabolic dysfunction, this dual-marker approach offers a practical tool for CAD risk stratification, particularly in high-risk populations. Further multicenter studies are needed to validate these findings and examine additional biomarker combinations to refine CAD risk models.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









