Safety and Efficacy of BioMime Sirolimus-Eluting Stent System in All-Comers Real-World Population With Coronary Artery Stenosis: MILES Global Registry
DOI:
https://doi.org/10.14740/cr1724Keywords:
BioMime SES, Bifurcation lesions, Chronic total occlusion, Coronary artery disease, Drug-eluting stent, Stent thrombosisAbstract
Background: This study evaluated the safety and efficacy of BioMime sirolimus-eluting stent (SES) system, with an ultra-low strut thickness (65 µm), in real-world all-comers population with coronary artery stenosis (CAD).
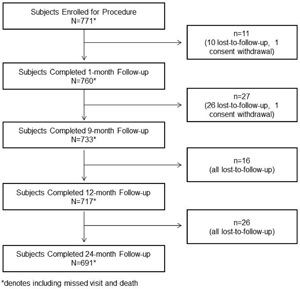
Methods: This was a post-marketing, multicenter, single-arm, observational clinical registry among patients undergoing intervention for CAD. Patients were clinically followed up at 1, 9, 12, and 24 months after the index percutaneous coronary intervention. Four major indications, namely long stents of > 30 mm, stents with diameters of 4 and 4.5 mm, bifurcation subgroup, and chronic total occlusion (CTO) were evaluated as pre-specified subsets.
Results: A total of 771 patients (1,079 treated lesions) from 23 sites were included in this study. The mean length and diameter of the implanted stents were 25.57 ± 9.35 mm and 3.00 ± 0.44 mm, respectively. The mean minimum lumen diameter before and after the procedure was 1.00 ± 1.69 mm and 2.96 ± 1.35 mm, respectively. The cumulative rates of major adverse cardiovascular events (MACEs) and stent thrombosis (ST) at 1, 9, 12, and 24 months were 1.05%, 3.13%, 4.04%, 5.64% and 0%, 0.13%, 0.28%, 0.28%, respectively. In a subset with > 30 mm long stents, the cumulative rate of MACEs was 0.4%, 4.6%, 5.12%, and 7.01% at 1, 9, 12, and 24 months, respectively. The corresponding rates of ST were 0%, 0.42%, 0.43%, and 0.44%, indicating constant rate of ST after 9 months. In a subset of 4 and 4.5 mm diameter stents, the cumulative rate of MACEs was high (0%, 6.25%, 6.25%, and 10.41%) at 1, 9, 12, and 24 months, respectively. However, there was no case of ST until 24 months. In patients with bifurcation lesions, the cumulative rates of MACEs and ST were 2.46%, 6.32%, 11.53%, 16.21% and 0%, 1.27%, 1.28%, 1.35% at 1, 9, 12, and 24 months follow-up. In patients with chronic total occlusion, the cumulative rates of MACEs and ST were 0.79%, 5.04%, 6.83%, 7.07% and 0%, 0.84%, 0.85%, 0.88% at 1, 9, 12, and 24 months, respectively, indicating constant rate of ST after 9 months.
Conclusions: The BioMime SES demonstrated good safety and efficacy outcomes at 24-month follow-up, with low rates of MACEs and ST in patients with CAD in the real-world setting.
Published
Issue
Section
License
Copyright (c) 2024 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









