Evaluation of a Point-of-Care N-Terminal Pro-Brain Natriuretic Peptide Assay for Heart Failure Management
DOI:
https://doi.org/10.14740/cr2117Keywords:
NT-proBNP, Point-of-care testing, Cardiovascular disease, Heart failure, BiomarkerAbstract
Background: N-terminal pro-brain natriuretic peptide (NT-proBNP) is a key biomarker in heart failure (HF) diagnosis and management. This study aimed to evaluate performances of the LumiraDx® NT-proBNP, a point-of-care testing (POCT) device, focusing on imprecision, method comparison, and clinical practicability.
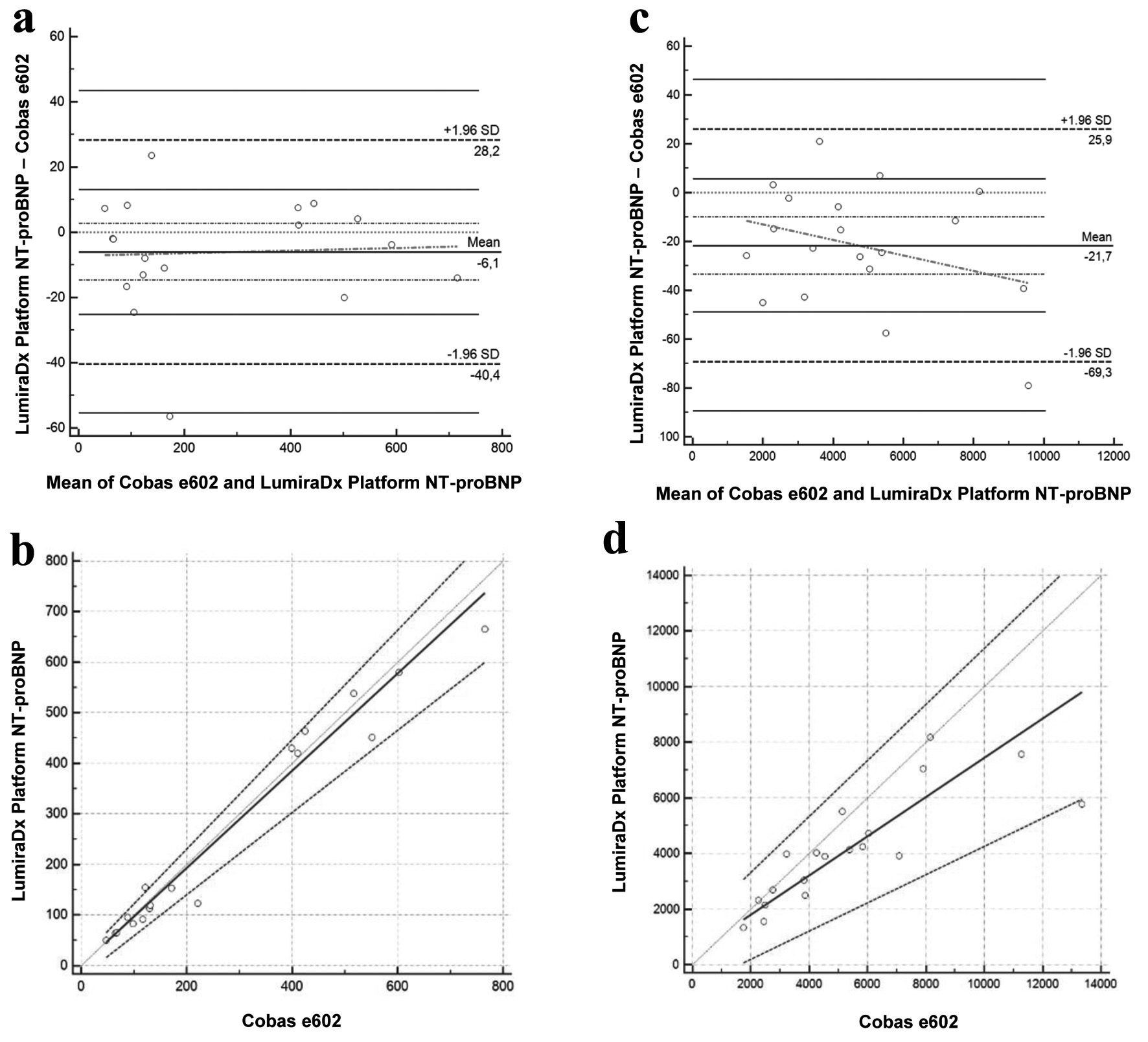
Methods: The LumiraDx® NT-proBNP test was assessed for imprecision across two reagent lots and compared with a reference laboratory method (Cobas e601) using 81 plasma samples. Method concordance was analyzed using Bland-Altman and Passing-Bablok regression. A user satisfaction survey evaluated its practicality in a clinical setting.
Results: For the first reagent lot, a coefficient of variation (CV) of 2.81% was observed, while for the second reagent lot, the CV was 5.4%. Method comparison revealed strong concordance with the reference method for NT-proBNP values < 1,000 ng/L. However, a significant bias was observed for values > 1,000 ng/L in the first lot, resolved in the second. User satisfaction surveys highlighted ease of use. Additionally, implementing the LumiraDx® NT-proBNP Platform resulted in a significant reduction in turnaround time, with an estimated 49 min saved in result reporting.
Conclusion: The LumiraDx® NT-proBNP POCT device demonstrates strong potential for HF management by combining rapid results, user-friendly operation, and sampling versatility. While biases at higher NT-proBNP levels warrant further standardization, this system represents a practical tool for decentralized HF care.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.









